- Media Preparation in Microbiology
- Water Quality Monitoring
- Environmental Monitoring
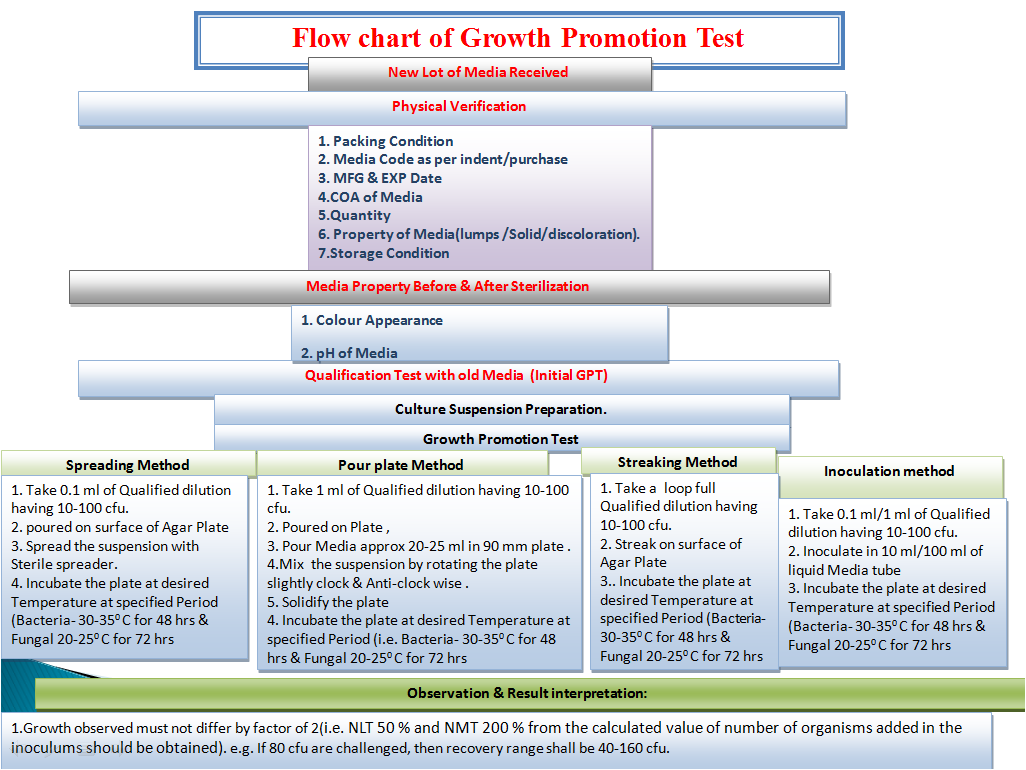
- Growth Promotion Test

- SOP for Sterility Test by Open Method


- Use of Bio ball Culture
- SOP for Culture Maintenance
- Alert & Action Limit Determination
- Qualification/Validation Concept
- Flow Chart of Microbial Limit Test
- Antibiotic Microbial Assay
- Culture Media
- Bacterial Endotoxin Test
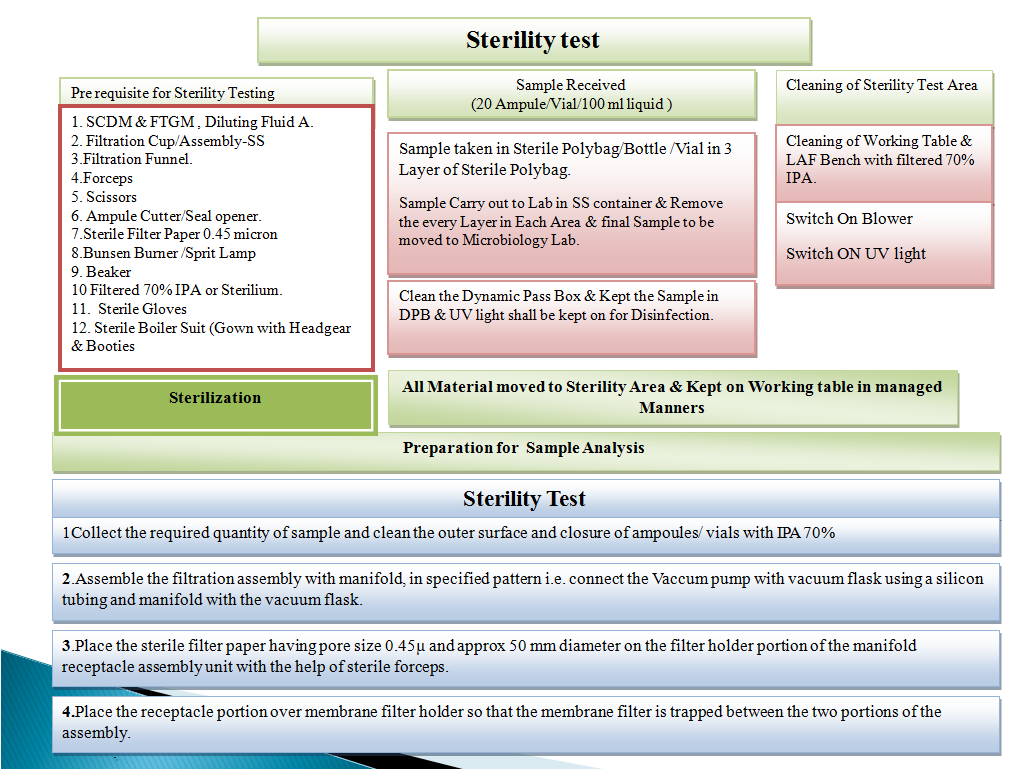
- Sterility Test
- Microbial Limit test of Non-Sterile Product
- History of Microbiology
- Microbiology Deviation
- Indian pharmacopea
-
Gram Staining
-
Culture Suspension technique 2023
-
Disinfectant efficacy test 2023
-
Incubator
-
Indian pharmacopea
-
Microbiology Deviation
-
History of Microbiology 2023
- Operation, Cleaning & Calibration of pH Meter
- Operation, Cleaning & Calibration of Micropipette:
- Operation ,Cleaning of Laminar Air Flow