(Good Laboratory Practices)GLP in Microbiology Laboratory:
INTRODUCTION :
Good laboratories Practices in a microbiology laboratory consist of activities that depend on several principles; aseptic technique, control of media, control of test strains, control of equipment, diligent recording and evaluation of data, and training of the laboratory staff Because of the known variability in microbiology data, reliability and reproducibility are dependent on the use of accepted methods and adherence to good laboratory practices.
Definition:
- Aseptic technique: Aseptic technique refers to the procedure used to avoid the introduction of pathogenic organisms into the vulnerable site. The prinicple aim of an aseptic technique is to protect the patient from contamination by pathogenic organisms.
- Seed Lot Technique: Seed Lot technique is used to provide a method for generation of a working culture no more than 5 passages removed from the original stock from the national repository.To provide a method for generation of a pure culture for laboratory work.To provide a method for generation of a traceable culture for laboratory investigations.
- SAL-Sterility Assurance Level: Sterility assurance level (SAL) is a term used in microbiology to describe the probability of a single unit being non-sterile after it has been subjected to the sterilization process. SAL also describes the killing of efficacy of a sterilization process. A very effective sterilization process has a very low SAL.
- Alert Level/Limit: Indicates when a process might have drifted from normal operating conditions. An investigation may be performed and corrective action may be implemented, but no action is required. It can be assumed that repetitive excursions above the alert level may be addressed as if it were an action level.
- Action Level/Limit: Indicates that a process has drifted from normal operating conditions. An investigation must be performed and corrective action must be implemented.
- Reference strains: Microorganisms defined at least to the genus and species level, catalogue and described according to its characteristics and preferably stating its origin. Normally obtained from a recognized national or international collection.
- Working culture: A primary subculture from a reference stock used as a reference in Microbiological analysis.
- Biological Safety Cabinet (BSC) : A ventilated cabinet with unidirectional HEFA- Filtered airflow and HEPA-Filtered exhaust to protect the worker from hazardous drugs. A BSC used to perform an activity must be capable of providing an ISO Class 5 environment for activity.
- Laminar airflow system (LAF) : A device or zone within a buffer area that provides an ISO Class 5 or better environment for sterile compounding/activities. The system provides a unidirectional HEFA-filtered airflow.
-
OBJECTIVE:
To provide a guideline for the Best Practices to be followed in a Microbiology Lab
-
SCOPE:
The procedure is applicable to all personnel working in Microbiology Lab.
-
RESPONSIBILITY:
- Microbiologists/Personnel Working in Microbiology Lab
- To follow the procedure while performing the Microbiological activities.
- To train the new employees during on job training
-
ACCOUNTABILITY :
- Head-Microbiology
-
- To ensure compliance to the procedures
- To ensure all the personnel are trained and adhere to the procedures
-
-
PROCEDURE:
-
Media Preparation and Quality Control
-
-
- Culture media are the basis for most of the microbiological tests, thus safeguarding the quality of media used is the critical for the execution of all the activities. Media preparation, storage and quality control testing can assure a consistent supply of high quality media.
- The correct composition of media if media is prepared from various ingredients should be followed. The instructions provided by the manufacturer should be followed properly if the dehydrated media or ready prepared media are used.
- Instructions provided like addition of additives, heating/sterilization, pH adjustment etc. should be followed meticulously in order to provide a consistent quality of media from a pack/batch/lot.
- A certificate of analysis from the Manufacturer of media with details of Batch/Lot No., Expiry date, Quality control testing details etc. should be maintained for each type/Batch/Lot of media/Ready to use /Ready to prepare media.
- Media should be stored under appropriate conditions e.g. Cool, dry, dark. All containers esp. of dehydrated media should be tightly closed and if the media are caked or cracked or show a change in the color then the media should not be used for testing purposes and should be discarded.
- Water of preparation of media should be free of any bactericidal, inhibitory or interfering substance and should be of a suitable microbiological quality. Preferably purified water should be used for the media preparation, but in some cases deionized or distilled water can be used. The quantity of water used for media preparation shall be recorded.
- A calibrated balance with appropriate weight range shall be used for weighing of the media and additives used.
- Clean weighing containers,materials and tools (eg. Spatula) should be used for media weighing and dispensing. Care should be taken to avoid any foreign contamination during weighing and dispensing. The media weights/component weights should be appropriately recorded.
- Dehydrated media should be properly dissolved prior to dispensing in various containers, may be be heating. Care should be taken to avoid overheating as all culture media to a greater or lesser extent are heat sensitive.
- Equipment (Water bath/Steam Pot) used for dissolution/preparation should be appropriate to allow for controlled heating,constant agitation, or mixing of media.
- Darkening of media (Maillard-type reaction or non enzymatic browning) is a general indication of overheating.Overheated media should not be used for Microbiological testing and should be disposed off.
- When adding supplements to the media, care should be taken to properly mix the supplements in media prior too sterilization/usage.
- Cleaned glassware’s only shall be used for preparation of media, ensure prior to usage that the glassware’s are free of any traces of debris, foreign matter and that the traces of detergent if used are thoroughly rinsed of using appropriate water quality.
- Sterilization of media should be performed using validated cycles or as specified by the manufacturer. Commercially available prepared media manufacturers should provide the method used for the sterilization. Manufacturer should provide if feasible the Sterility Assurance level (SAL) obtained during sterilization using Biological Indicator.
- Autoclaving by steam sterilization is preferred method for sterilization of media, except where boiling is recommended by manufacturer to avoid deterioration of heat labile components of media. The steam sterilization cycle should be validated to ensure proper heat distribution for selected loads and volumes and should ensure sterility and effective Growth Promotion test of media.
- The load configuration of the autoclave will influence the rate of heating,longer cycles may be required. The sterilization cycle will be dependent on the media volume and autoclave load. Sterilization cycle time in which the autoclave is slow to come up to temperature may result in overheating of the media.
- The pH of each batch of medium shall be confirmed after it has cooled to room temperature preferably 25ºC by aseptically withdrawing a sample for testing if required. The pH of the media should be in a range as specified by the manufacturer or as per the validated process. A flat pH probe is recommended for agar surfaces, and an immersion probe is recommended for liquids.
- Repartition of media after sterilization should be performed under unidirectional airflow (UDAF) to minimize potential for environmental contamination. This should be considered a minimum requirement for media to be used in relation to sterile product testing. This includes the cooling of media, as container lids will need to be removed during cooling to prevent build-up of condensation.
- Prepared media should be checked for the following parameters as applicable for the sterilized tubes/plates
-
- Cracked containers or lids
- Unequal filling of containers
- Dehydration resulting in cracks or dimpled surfaces on solid medium
- Excessive darkening or color change
- Crystal formation form possible freezing
- Excessive number of bubbles
- Microbial contamination
- Lot number and expiry date checked and recorded
- Sterility of the media
-
-
-
Media Storage

-
-
- Media should be transported and stored in conditions that minimize the loss of moisture, control the temperature and provide mechanical protection to the prepared media.
- Media prepared in house should be stored under validated conditions. Do not store agar at or below 0ºC, as freezing could damage the gel structure. Protect stored media from exposure to light and excessive temperatures.
- Media should be labeled properly with batch/lot numbers, preparation and expiry dates and media identification as applicable. Dehydrated/ Ready Prepared Media should be stored as per the manufacturers recommendation.
- If stored for prolonged period of time the agar plates should be placed into a sealed package or container to retard moisture loss
- Re-melting of an original container of solid media should be performed only once to avoid media quality being compromised by overheating or potential contamination. It is recommended that re-melting be performed in a heated water bath or by using free flowing steam.
- The use of microwave ovens and heating plates is common, but care should be taken to avoid damaging media by overheating and to avoid the potential injury to laboratory personnel from glass breakage and burns.
- The molten agar medium should be held in a monitored water bath at a temperature of 45-50ºC for not more than 8 hours. Wiping of the exterior of the container should be done prior to usage of container under LAF for pouring into sterile containers/plates etc.
- Disposal of used culture media as well as expired media should be done as per site specific SOP, but the media should only be discarded after proper decontamination either through sterilization or usage of chemical disinfectants.
-
-
Testing of media
-
-
- QC testing is essential as manufacturers of media attempt to standardize raw materials from biological sources, but must constantly deal with unavoidable differences in raw materials obtained from natural sources, and therefore lot to lot variability is observed
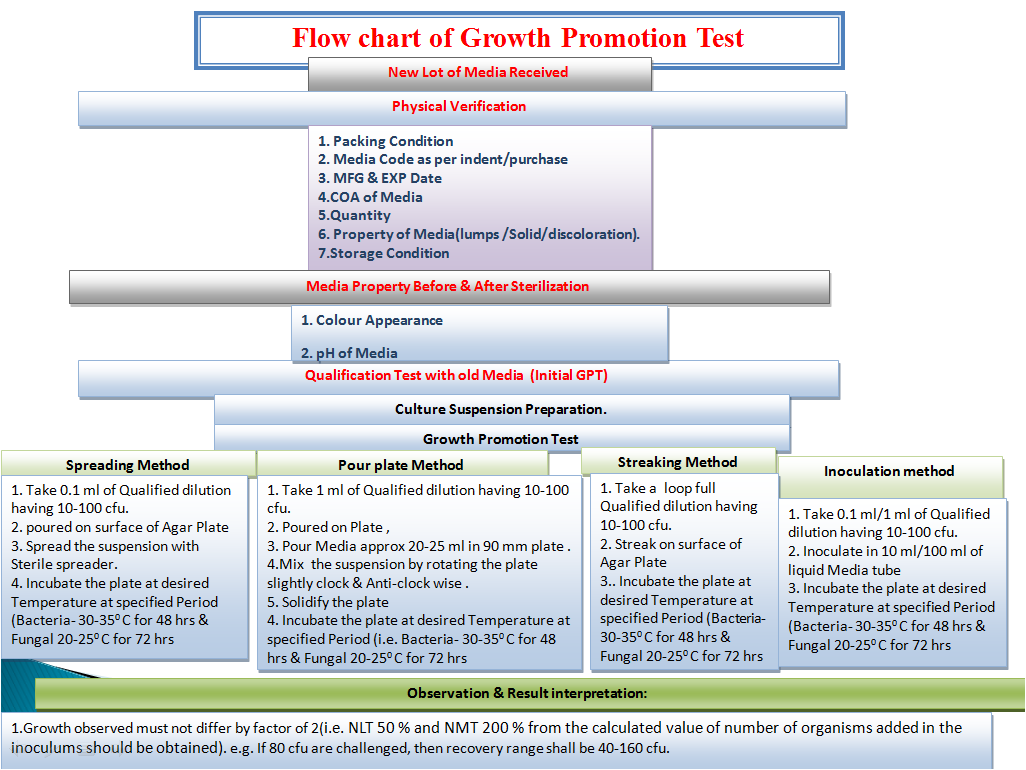
- Quality control testing should be performed in laboratory on all prepared media. Tests routinely performed on in house media are pH, growth promotion and periodic stability checks to confirm the expiry dating
- When the in house prepared media are properly prepared and sterilized as per validated methods, then each batch testing can be limited to each incoming lot of dehydrated media, unless otherwise instructed by the relevant compendial testing method
- If the media preparation is not validated, each prepared media lot should be subjected to growth promotion testing. The test organisms used for the testing should be as per the Procedure for Media Preparation SOP
- Expiry of the media should have supporting growth promotion testing to indicate that the performance of the media still meets acceptance criteria up to and including the expiration date. The length of shelf life of the batch of media depends upon the stability of the ingredients and formulation under specified conditions as well as the storage containers.
- An investigation should be initiated if Growth promotion testing do not meet the acceptance criteria to identify the cause of the failure. The investigation should include the corrective action plan to prevent the recurrence of the problem. Any nonconforming lot should not be used if assignable cause or corrective action to non growth promotion is undetermined.
- Quality control testing for some of the reagents routinely used in the labs for diagnostic purposes should be performed e.g. Gram Stain kit and oxidase test reagent/discs. Select correct quality control standard microorganisms, and perform the testing prior to unknown sample diagnostic testing as per the manufacturer’s instructions.
- Special care should be taken with media that is used in environmental monitoring studies. Media used for environmental monitoring of critical areas should preferably be double-wrapped and terminally sterilized. If terminal sterilization is not performed, media should be subjected to pre-incubation and 100% inspection prior to use within a critical area. This will prevent extraneous contamination from being carried into controlled environments and will prevent false-positive results. A raised agar level for surface contact plates should be verified.
- Growth promotion test shall be performed after completion of pre-incubation of media plates used for environment monitoring of critical areas if the plates are not terminally sterilized.
-
-
Maintenance of Microbial Cultures
-
-
- Biological cultures can be the most delicate standards to handle because their viability and characteristics are dependent on adequate handling and storage.
- Standardizing and handling and storage of cultures by the user laboratory should be done in a way that will minimize the opportunity for contamination or alteration of growth characteristics
- The careful and consistent treatment of stock cultures is critically important to the consistency of microbiological test results.
- Cultures for use in various test should be acquired from a national culture collection.
- They can .be acquired frozen, freeze dried, on slants or in ready to use forms. Confirmation of the purity of the culture and the identity of the culture should be performed prior to its use in and testing
- Ready to use cultures may require confirmation of purity, identity and inoculums size.
- This confirmation of identity for commonly used laboratory strains should ideally be done at the level of genotypic analysis (i.e., DNA fingerprinting, 16S r RNA gene sequencing, or PCR analysis using suitably validated probes). Or a suitable methods to determine the genus and the species of the organism (e.g. Biochemical Enzymatic substrate kit)
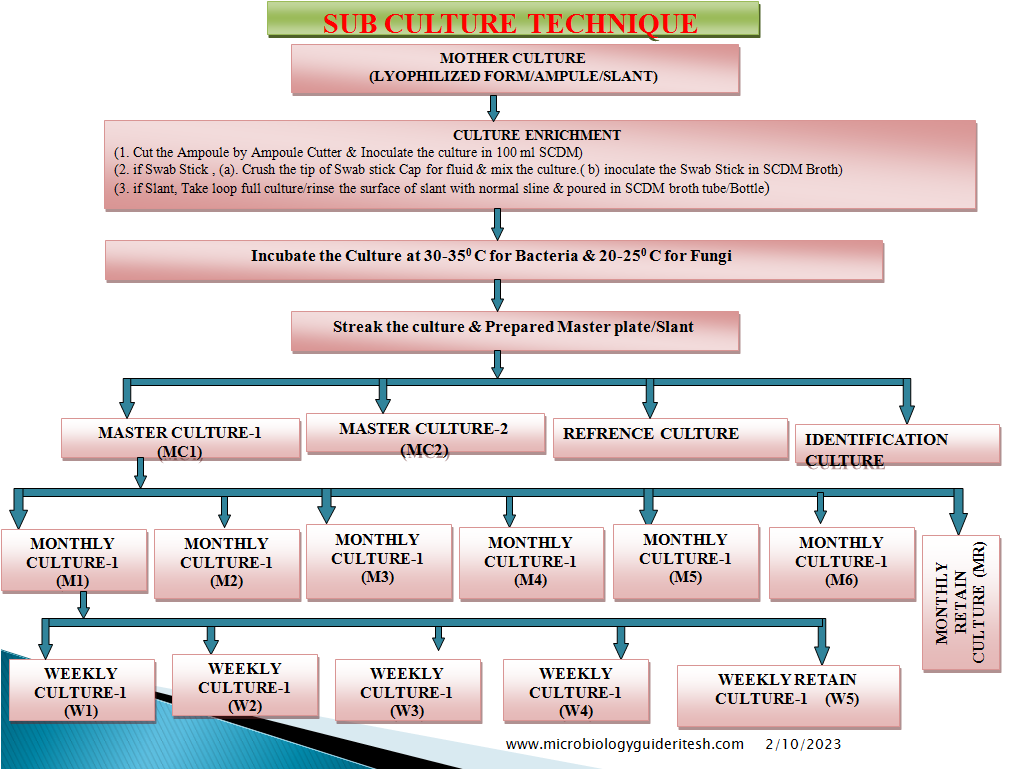
- Preparation and resuscitation of cultures should follow the instructions of the supplier or a validated, established method. The “Seed-Lot” technique is recommended for storage of stock cultures.
- The original sample from the national culture collection is resuscitated and grown in an appropriate medium. Aliquots of this stock culture (the first transfer or passage) are suspended in a cryoprotective medium, transferred to vials, and frozen at –30ºC or below, until use. If stored at –70ºC, or in lyophilized form, strains may be kept indefinitely.
- The frozen stocks of cultures can be used to inoculate monthly or weekly working cultures.
- Once opened, do not refreeze unused cell culture suspensions after culturing a working suspension. The unused portion should be discarded to minimize the risk of loss of viability and contamination of the stock.
- The number of transfers of working control cultures should be tracked to prevent excessive sub culturing that increases the risk of phenotypic alterations.
- One passage is defined as the transfer of organisms from a viable culture to a fresh medium with growth of the microorganisms. Any form of sub culturing is considered to be transfer/passage.
- 1.1.14 Total number of passages shall not exceed five from the original culture.
-
-
Maintenance of Laboratory equipment
-
- Most equipment’s (incubators, water baths and steam sterilizers) are subject to standard validation practices of incoming qualification, operational qualification and performance qualification
- Additionally periodic calibration is required (generally annually). New equipment critical to the operation of the laboratory should be qualified according to a protocol approved by the respective Quality assurance
- Instruments (e.g. pH meters, balances, spectrophotometers) used in a microbiology laboratory should be calibrated on a regular schedule and tested to verify performance on a routine basis. The frequency of calibration and performance verification will vary based on the type of instrument and the importance of that equipment to the generation of data in the laboratory.
- Laboratories should have a separate autoclave for decontamination. However, in exceptional cases one autoclave may be acceptable provided that extensive precautions are taken to separate decontamination and sterilization loads, and a documented cleaning programme is in place to address both the internal and external environment of the autoclave.
- Microbiology laboratories should carry out initial verification of volumetric equipment (automatic dispensers, dispenser/diluters, mechanical hand pipettes and disposable pipettes) and then make regular checks, as appropriate, to ensure that the equipment is performing within the required specification. Initial verification should not be necessary for glassware which has been certified to a specific tolerance.
- Equipment should be checked for the accuracy of the delivered volume against the set volume (for several different settings in the case of variable volume instruments) and the precision of the repeat deliveries should be measured.
- Hygrometers should be calibrated, the calibration being traceable to national or international standards.
- When centrifuges are used in test procedures, an assessment of the rotations per minute (RPM) should be made. Where it is critical, the centrifuge should be calibrated.
-
-
Laboratory Layout and operations
-
- Laboratory layout and design should carefully consider the requirements of good microbiological practices and laboratory safety.
- It is essential that cross contamination of microbial cultures be minimized to the greatest extent possible and it is also important that microbiological samples be handled in an environment that makes contamination highly unlikely . Where possible segregate the activities involving live bacterial cultures from clean/aseptic activities which do not involve live bacterial cultures.
- Areas in which environmental or sterile product samples are handled and incubated should be maintained completely free of live cultures, if possible. If complete separation of live and clean culture zones cannot be accomplished, then other barriers and aseptic practices should be employed to reduce the likelihood of accidental contamination. These barriers include protective clothing, sanitization and disinfection procedures, and biological safety cabinets designated for clean or aseptic operations only.
- Procedures for handling spills or mishaps with live cultures should be in place, and all relevant technical personnel should be trained regarding these methods. Some samples will demonstrate microbial growth and require further laboratory analysis to identify the contaminants. When growth is detected, the sample should be taken from the clean section of the laboratory to the live culture section without undue delay.
- Sub culturing, staining, microbial identification, or other investigational operations should be undertaken in the live culture section of the laboratory. If possible, any sample found to contain growing colonies should not be opened in the clean zone of the laboratory. Careful segregation of contaminated samples and materials will reduce false-positive results.
- Staff engaged in sampling activities should not enter or work in the live culture handling section of a laboratory unless special precautions are taken, including wearing protective clothing and gloves, and careful sanitization of hands upon exiting. Ideally, staff assigned to sampling activities, particularly those in support of aseptic processing, should not work in the vicinity of live culture laboratory operations.
- All microbiological samples should be handled using aseptic techniques, including those taken in support of non sterile products. If possible all microbiological samples should be taken under full aseptic conditions in specialized sampling areas.
- It is important to consider that microbial contamination of samples, which leads to false-positive results, is always possible unless careful aseptic precautions are taken. Facilities should be designed so that raw material and excipient sampling can be done under controlled conditions, including proper gowning and sterilized sampling equipment. It may not always be possible to sample utility systems, such as water systems, under full aseptic conditions; however, it should be noted that when samples are not taken aseptically, their reliability is inevitably compromised.
- Environmental sampling methods should require minimal aseptic handling in loading and unloading sampling instruments. Whenever possible, sampling equipment should be loaded with its microbiological recovery media in the environment that is to be sampled.
- All testing in laboratories used for critical testing procedures, such as sterility testing of final dosage forms, bulk product, handling of seed cultures should be performed under controlled conditions.
- There should be separate air supply to laboratories and production areas. Separate air-handling units and other provisions, including temperature and humidity controls where required, should be in place for microbiological laboratories. The air supplied to the laboratory should be of appropriate quality and should not be a source of contamination.
- Access to the microbiological laboratory should be restricted to authorized personnel. Personnel should be made aware of:
- the appropriate entry and exit procedures including gowning;
- the intended use of a particular area;
- the restrictions imposed on working within such areas;
- the reasons for imposing such restrictions; and
- the appropriate containment levels.
- Consideration should be given to designing appropriate classified areas for the operations to be performed within the microbiology laboratory.The classification should be based on the criticality of the product and the operation being carried out in the area. Sterility testing should be performed under the same class as used for sterile/aseptic manufacturing operations.
- The sterility testing should be carried out within a Grade A unidirectional airflow protected zone or a bio safety cabinet (if warranted), which should be located within a clean room with a Grade B background. Care should be taken with the design of the facility layout and room airflow patterns, to ensure that the unidirectional airflow patterns are not disrupted.Air supplied to Grade A and B zones should be via terminal HEPA filters
- Entry to the clean room should be via a system of airlocks and a change room where operators are required to don suitable clean-room garments. The final change room should be under “at rest” conditions of the same grade as the room it serves. Change rooms should be of adequate size for ease of changing. There should be clear demarcation of the different zones.
-
-
Training of personnel

Speaker at Presentation/training. personnel in Trraining Hall.
-
-
- Each person engaged in all phases of pharmaceutical microbiology operations should have the education, training and experience to do his or her job.
- The demands of microbiological testing require that the core educational background of the staff , supervisors and managers be in microbiology or a closely related biological science.
- They should be assigned responsibilities in keeping with their level of skill and experience.
- A coherent system of operating procedures is necessary to run the microbiology laboratory. These procedures serve two purpose in a training program.
- Firstly, these SOPs describe the methodology that the microbiologist will follow to obtain accurate and reproducible results, and so serve as the basis for training.
- Secondly, by tracking the procedures in which a particular microbiologist has demonstrated proficiency, the procedure number or title also serves to identify what training the microbiologist has received specific to his or her job function.
- Training curricula should be established for each laboratory staff member specific for his or her job function. They should not independently conduct a microbial test until they are qualified to run the test.
- Training records should be current, documenting the microbiologists training in the proper revision to the particular SOP.
- Periodic performance assessment of each analyst is required to ensure data quality. This performance testing should provide evidence of competency in the core activities of the microbiology laboratory such as hygiene, plating, aseptic technique, documentation and others as suggested by the microbiologists job function.
- Microbiologists with supervisory or managerial responsibilities should have appropriate education, experience and in house training in supervisory skills, laboratory safety, scheduling, laboratory investigations, technical report writing, relevant SOPs and other critical aspects of the company’s processes as suggested in their role of directing a laboratory function.
- Operators should be trained and certified in gowning procedures with training records maintained.
- Microbiological sampling ,Sample handling and transportation
- Any disinfection processes used in obtaining the sample (e.g.disinfection of sample points) should not compromise the microbial level within the sample.
- Transport and storage of samples should be under conditions that maintain the integrity of the sample (e.g. chilled or frozen where appropriate). Testing of the samples should be performed as soon as possible after sampling. For samples where a growth in the microbial population during transport and storage is possible it should be demonstrated that the storage conditions, time and temperature, will not affect the accuracy of the testing result. The storage conditions should be monitored and records kept. The responsibility for transport, storage between sampling and arrival at the testing laboratory should be clearly documented.
- Sampling should only be performed by trained personnel. It should be carried out aseptically using sterile equipment. Appropriate precautions should be taken to ensure that sample integrity is maintained through the use of sterile sealed containers for the collection of samples where appropriate. It may be necessary to monitor environmental conditions, for example, air contamination and temperature, at the sampling site. Time of sampling should be recorded, if appropriate.
- The laboratory should have procedures that cover the delivery and receipt of samples and sample identification. If there is insufficient sample or the sample is in poor condition due to physical deterioration, incorrect temperature, torn packaging or deficient labeling, the laboratory should consult before deciding whether to test or refuse the sample.
- Samples awaiting testing should be stored under suitable conditions to minimize changes to any microbial population present. Storage conditions should be validated, defined and recorded.
- The packaging and labels of samples may be highly contaminated and should be handled and stored with care so as to avoid any spread of contamination. Disinfection processes applied to the outer container should not affect the integrity of the sample.
- Laboratory sample portions that are known to be contaminated should be decontaminated prior to being discarded
-
-
Documentation
-
- Documentation should be sufficient to demonstrate that the testing was performed in a laboratory and by methods that were under control. This includes, but not limited to documentation for the following.
-
- Microbiologist training and verification of proficiency
- Equipment validation, calibration and maintenance
- Equipment performance during test
- Media preparation and its growth promotion capabilities
- Media inventory and control testing
- Data and calculation verification
- Reports reviewed by reviewer
- Investigation of Microbial Data Deviations (if required)
-
- If the result of the enumeration is negative, it should be reported as “not detected for a defined unit” or “less than the detection limit for a defined unit”. The result should not be given as “zero for a defined unit” unless it is a regulatory requirement. Qualitative test results should be reported as “detected/not detected in a defined quantity or volume”. They may also be expressed as “less than a specified number of organisms for a defined unit” where the specified number of organisms exceeds the detection limit of the method and this is acceptable. In the raw data the result should not be given as zero for a defined unit unless it is a regulatory requirement. A reported value of “0” may be used for data entry and calculations or trend analysis in electronic databases
- Documentation should be sufficient to demonstrate that the testing was performed in a laboratory and by methods that were under control. This includes, but not limited to documentation for the following.
-
-
Maintenance of Laboratory Records
-
- Proper recording of data and studies is critical to the success of microbiology laboratory. The various tests should be performed as per the SOP and the laboratory notebook should provide a record of all critical details needed to confirm the integrity of the data. At a minimum, the laboratory Test Raw Data sheet should include the following:
- Date
- Material tested
- Microbiologist’s name and Signature
- Procedure number
- Document test results
- Deviations (if any)
- Documented parameters (equipment used, microbial stock cultures used, media lots used etc.)
- Management/Second review signatures
- Every critical piece of equipment should be noted in the test data sheet an the all should be on a calibration schedule documented by SOP and maintenance records
- Where appropriate, log books/forms should be available and supportive of the laboratory logbook records. Equipment temperatures (incubators, autoclaves) should be recorded and traceable.
- The details of the SOP governing the test should be clearly noted in the test data sheet and changes in the data should be crossed off with a single line and initialed. Original data should not be erased or covered by any means
- Test results should include the original plate counts, allowing a reviewer to recalculate the derived final results. Methods for data analysis should be detailed in site specific SOP’s.
- Proper recording of data and studies is critical to the success of microbiology laboratory. The various tests should be performed as per the SOP and the laboratory notebook should provide a record of all critical details needed to confirm the integrity of the data. At a minimum, the laboratory Test Raw Data sheet should include the following:
-
-
Interpretation of Assay Results
-
- Analytical microbiological assay results can be difficult to interpret for several important reasons:
- Microorganisms are ubiquitous in nature and common environmental contaminants, particularly organisms associated with humans predominate in many types of microbiological analysis.
- The analyst has the potential to introduce contaminating organisms during sample handling or processing in the laboratory
- Microorganism may not be homogeneously distributed within a sample or an environment.
- Microbiological assays are subject to considerable variability of outcome. Therefore , minor differences from an expected outcome may not be significant. Due to the variability in assays, utmost care should be taken to avoid exogenous contamination.
- Results must be interpreted from a broad microbiological perspective considering not only the nature of the contaminant, but the likelihood of that organisms surviving in the pharmaceutical ingredient, excipient and/or environment under test. In addition, the growth characteristics of the microorganisms should be considered.
- When result are observed that do not conform to a compendial monograph or another established quantitative target, an investigation into the finding is required.
- There are generally two distinct reasons for the observation of microbial contamination that does not comply with a target or requirement: There may be either a laboratory error or laboratory environmental conditions that produced an invalid result, or the product contains a level of contamination or specific types of contaminants outside established levels or limits.
- A full and comprehensive evaluation of the situation surrounding the result should be undertaken. All microbiological conditions or factors that could bring about the observed condition should be fully considered, including the magnitude of the excursion compared to established limits or levels. It is critical to know if the finding is statistically significant in light of assay variability.
- The laboratory environment, the protective conditions in place for sampling, historical findings concerning the material under test, and the nature of the material, particularly with regard to microbial survival or proliferation in contact with the material, should be considered in the investigation.
- In addition, interviews with the laboratory analyst(s) may provide information regarding the actual conduct of the assay that can be valuable in determining the reliability of the result and in determining an appropriate course of action. If laboratory operations are identified as the cause of the nonconforming test outcome, then a corrective action plan should be developed to address the problem(s). Following the approval and implementation of the corrective action plan, the situation should be carefully monitored and the adequacy of the corrective action determined.
- If assay results are invalidated based upon the discovery of an attributable error, this action must be documented. Laboratories also should have approved procedures for confirmatory testing (retesting), and if necessary, re-sampling where specific regulatory or compendial guidance do not govern the conduct of an assay investigation.
-
-
Environment Monitoring of Testing areas
-
- Environmental microbiological monitoring should reflect the facility used (room or UAF/LAF/BSC) and include a combination of air and surface sampling methods appropriate to the facility, such as:
-
- active air sampling;
- settle (exposure) plates;
- surface contact — replicate organism detection and counting (RODAC)
- plates, swabs or flexible films;
- operators glove prints (where applicable).
-
- Microbial environmental monitoring of the sterility test zone should be performed during every work session under operational (dynamic) conditions.
- There should be written specifications, including appropriate alert and action limits for microbial contamination.
- Environmental microbiological monitoring should reflect the facility used (room or UAF/LAF/BSC) and include a combination of air and surface sampling methods appropriate to the facility, such as:
-



-
Validation of Test Methods.
-
- Standard (pharmacopoeial) test methods are considered to be validated. However, the specific test method to be used by a specific laboratory for testing of a specific product needs to be shown to be suitable for use in recovering bacteria, yeast and mold in the presence of the specific product. The laboratory should demonstrate that the performance criteria of the standard test method can be met by the laboratory before introducing the test for routine purposes (method verification) and that the specific test method for the specific product is suitable (test method suitability including positive and negative controls).
- Test methods not based on compendial or other recognized references should be validated before use. The validation should comprise, where appropriate, determining accuracy, precision, specificity, limit of detection,limit of quantitation, linearity and robustness. Potentially inhibitory effects from the sample should be taken into account when testing different types of sample. The results should be evaluated with appropriate statistical methods, e.g. as described in the national, regional or international pharmacopoeias.
-
-
Interpretation of Alert and Action limits

-
-
- For all the monitoring activities, such as environmental monitoring activity, compressed air monitoring activity, water analysis or any other monitoring activity with defined frequency has to be established with alert and action limits.
- Alert Limit: Microbial levels specified in the SOP , which are a specific for a given facility and are established on the basis of baseline developed under monitoring program. Alert levels are always lower than the action level, results exceeding alert level gives early warning of the potential drift from natural operating conditions they may trigger appropriate scrutiny and follow up to address the potential problem and they may not be necessary ground for definitive corrective actions.
- Action Limit: Microbial level specified in the SOP based on the guideline values. Action level results equal to or exceeding action level, which should trigger appropriate investigation and corrective action based on the investigation.
-
-
Microbiology Practice in routine analysis
-
- Labeling of microbiological analysis plates/tubes shall be done as per Procedure For Labeling Of Dispensed Microbiological Media Containers
- MET &TSM analysis plates shall be labeled at the bottom of the plates and near neck of the tubes with the media batch no., product name & batch no., stage/condition of testing if any, date of testing.
- Environmental monitoring plates/Compressed air monitoring shall be labeled by marker pen with date of monitoring, media batch no. Room no, location no/name of location/sampling location or area at the bottom of the plate
- Culture tubes shall be labeled with culture inoculation date, name of culture, Passage no, near the neck of the tubes.
- Inoculums preparation plates shall be labeled by marker with date of inoculation, media batch no. Name of culture, dilution no.
- Isolate plates shall be labeled with marker pen with the type of isolate, location name, date of monitoring, media batch no.
- Personnel monitoring plates shall be labeled by marker with person name, date of monitoring, media batch no, monitoring location name.
- Water analysis plates shall be labeled at the bottom of the plates and near neck of the tubes with the media batch no., sampling location ID/name, date of sampling/testing
- Sterility analysis tubes/canisters shall be labeled near neck of the tubes/Face of the canister with the media batch no., product name & batch no., stage/condition of testing if any, date of testing.
- To have a better traceability for the analysis plates and tubes/canisters etc. Racks/trays of incubators shall be differentiated day/date wise and all analyst/microbiologist shall incubate the plates and tubes of analysis in respective racks/trays. The differentiation can be done in trays for various activities separately.
- On day/date of observation the plates due for release shall be removed from the incubator and the results shall be recorded in Lab data sheets and second qualified microbiologist shall verify the results recorded.
- Note: While reading plates and tubes, if second qualified microbiologist is not available, store the plates and tubes in cold chamber for maximum of 72 hrs. Second verifier shall complete verification within 72 hrs.
- If any differences found in results of observer and verifier, supervisor shall review and conclude the results to be reported and the supervisor shall acknowledge the same on lab data sheet.
- The time of recording for incubation/observation shall be noted form validated satellite clock only.
- Only respective analyst/microbiologist responsible for plate reading shall transfer the observed plates and tubes to the decontamination area.
- All samples which are under analysis shall be placed in designated area inside the Microbiology Lab.
- Doors to laboratories shall not be left open.
- Open wounds, cuts, scratches and grazes should be covered with water proof dressing.
- Papers and reports shall be kept in separate areas away from the bio hazardous materials work area
- Eating, drinking,smoking and storing of either food, personnel belongings or utensils in laboratory is prohibited.
- Applying cosmetics and inserting or removing contact lenses are not permitted in the laboratory.
- Wearing jewelry is not recommended while working under the laminar air flow hood,in sterile area and in bio safety cabinets.
- Talking to personnel while working under the UAF/LAF/BSC is not acceptable
- Good microbiology practices intended to avoid the release of infectious agents are to be employed.
- Media containing anti metabolites or inhibitors shall be prepared using dedicated glassware, as carryover of these agents into the other media/samples may lead to false negatives.
- The recommended counting range for plates inoculated with known concentration of culture is as below.
- IF counting Recommended countable range
- Bacteria and Yeast on 90 mm petriplate 25-250 cfu/plate
- Mold on 90 mm petriplate 8-80 cfu/plate
- Bacteria,Yeast and mold on petriplate with 47 mm diameter membrane filter (used during membrane filtration technique) 20-80 cfu/filter face on a plate
-
6.0 ABREVIATION(S):
UAF : Uni directional Air Flow system
LAF : Laminar Air Flow system
BSC : Bio safety Cabinet
SAL : Sterility Assurance Level
cfu : Colony Forming units
mm : millimeter
MET : Microbial Enumeration test
TSM : Test for Specified microorganisms
SOP : Standard Operating Procedure
RODAC : Replicate Organism Detection and counting
DNA : Deoxy ribo nucleic Acid
RNA : Ribo nucleic Acid
PCR : Polymerase chain reaction
HEPA : High Efficiency Particulate Air
7. ANNEXURES:
NIL
8. DISTRIBUTION:
MB
9. REFERENCES:
NA
10. REVISION HISTORY:
New




No doubt.
I agree with told all above. We can communicate on this theme. Here or in PM.
It is a pity, that now I can not express – I am late for a meeting. I will return – I will necessarily express the opinion on this question.
Certainly. All above told the truth. Let’s discuss this question. Here or in PM.
I am sorry, that has interfered… I understand this question. I invite to discussion.
It is interesting. You will not prompt to me, where to me to learn more about it?
This is really interesting, You’re a very skilled blogger. I’ve joined your feed and look forward to seeking more of your magnificent post. Also, I’ve shared your site in my social networks!
obviously like your website but you need to test the spelling on quite a few of your posts Several of them are rife with spelling problems and I to find it very troublesome to inform the reality on the other hand Ill certainly come back again
Somebody essentially lend a hand to make significantly articles Id state That is the very first time I frequented your website page and up to now I surprised with the research you made to make this actual submit amazing Wonderful task
I do not even know how I ended up here but I thought this post was great I do not know who you are but certainly youre going to a famous blogger if you are not already Cheers
Fantastic site Lots of helpful information here I am sending it to some friends ans additionally sharing in delicious And of course thanks for your effort
What i do not understood is in truth how you are not actually a lot more smartlyliked than you may be now You are very intelligent You realize therefore significantly in the case of this topic produced me individually imagine it from numerous numerous angles Its like men and women dont seem to be fascinated until it is one thing to do with Woman gaga Your own stuffs nice All the time care for it up
you are in reality a just right webmaster The site loading velocity is incredible It seems that you are doing any unique trick In addition The contents are masterwork you have performed a wonderful task on this topic
Hello! I just would like to give a huge thumbs up for the great info you have here on this post. I will be coming back to your blog for more soon.
Hi, Neat post. There is a problem with your web site in internet explorer, would check this… IE still is the market leader and a good portion of people will miss your excellent writing due to this problem.
Youre so cool! I dont suppose Ive learn anything like this before. So nice to search out anyone with some authentic ideas on this subject. realy thanks for starting this up. this website is something that’s needed on the web, someone with just a little originality. helpful job for bringing something new to the internet!
I am lucky that I found this website, exactly the right info that I was looking for! .
Perfect work you have done, this website is really cool with wonderful information.
Techno rozen Hi there to all, for the reason that I am genuinely keen of reading this website’s post to be updated on a regular basis. It carries pleasant stuff.