Microbial Limit Test:
The microbial limit test is a procedure used in the pharmaceutical and other industries to determine the presence and quantity of microorganisms in a sample. It is an essential part of ensuring the safety and quality of pharmaceutical products, as well as other products intended for human consumption.
The test is conducted to detect the presence of objectionable microorganisms, including bacteria, yeast, and molds, in a sample. These microorganisms may be harmful to human health or cause product spoilage. The microbial limit test helps evaluate the effectiveness of preservation methods and the overall microbiological quality of the product.
Here are the key steps involved in conducting a microbial limit test:
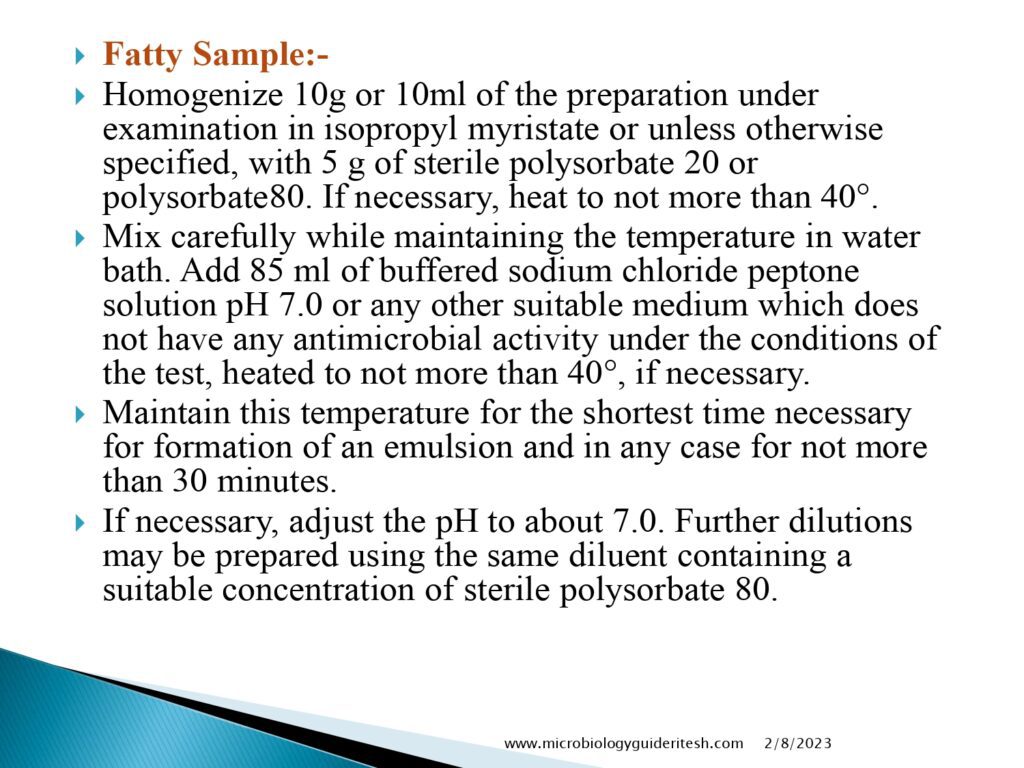
- Sample Preparation: The sample under consideration is collected and prepared according to specified procedures. It may involve dilution and filtration, depending on the nature of the sample.
- Inoculation: The prepared sample is then introduced into specific growth media that support the growth of different microorganisms. These media are chosen based on the type of microorganisms expected in the sample.
- Incubation: The inoculated media are incubated at suitable temperatures to allow the growth of microorganisms. Incubation times can vary depending on the microorganisms being tested.
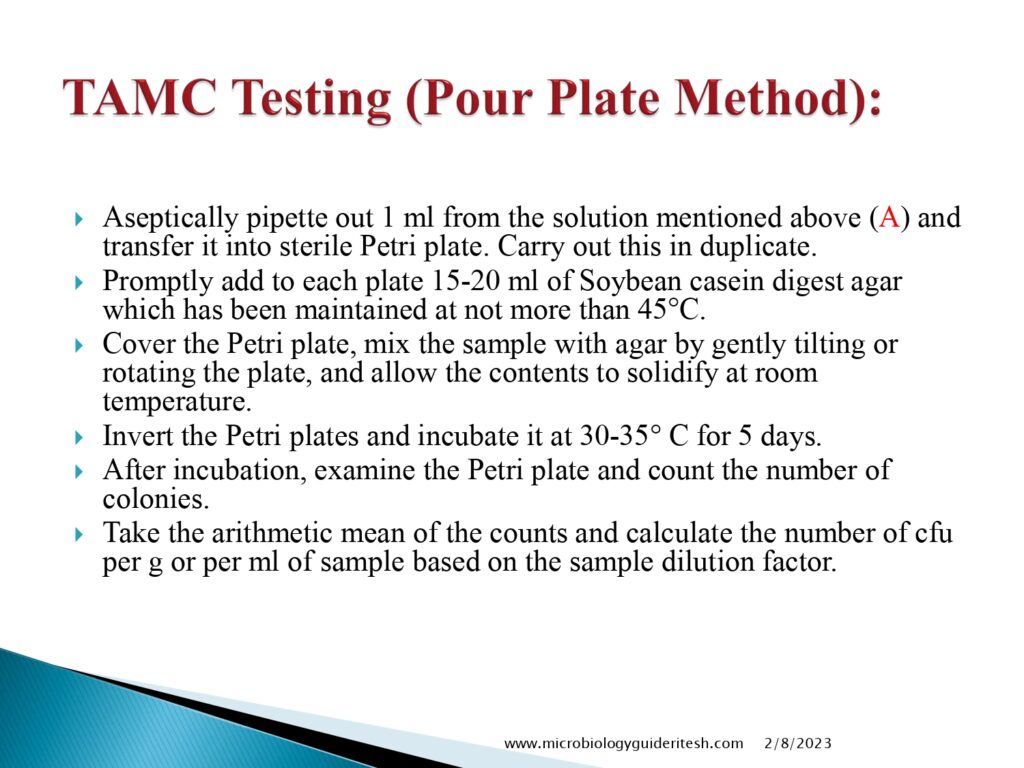
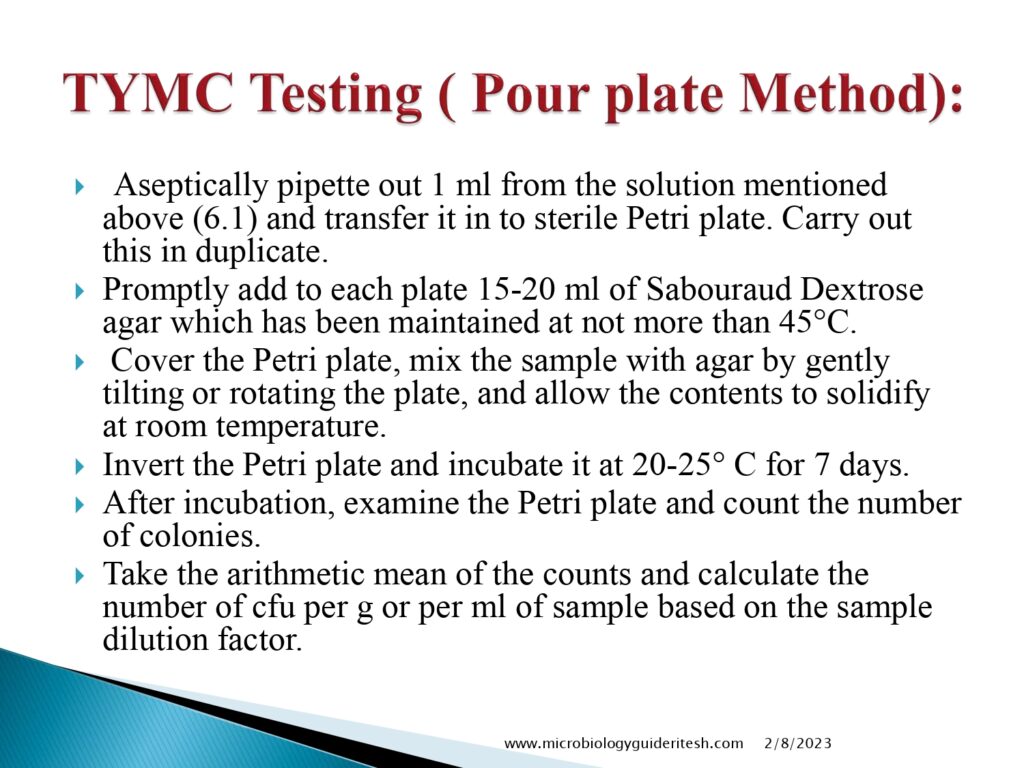
- Enumeration: After the incubation period, the colonies of microorganisms that have grown on the media are counted. This step helps determine the total viable microbial count in the sample.
- Identification: In some cases, further tests are conducted to identify the microorganisms present. These tests may include biochemical assays, microscopic examination, or genetic analysis.
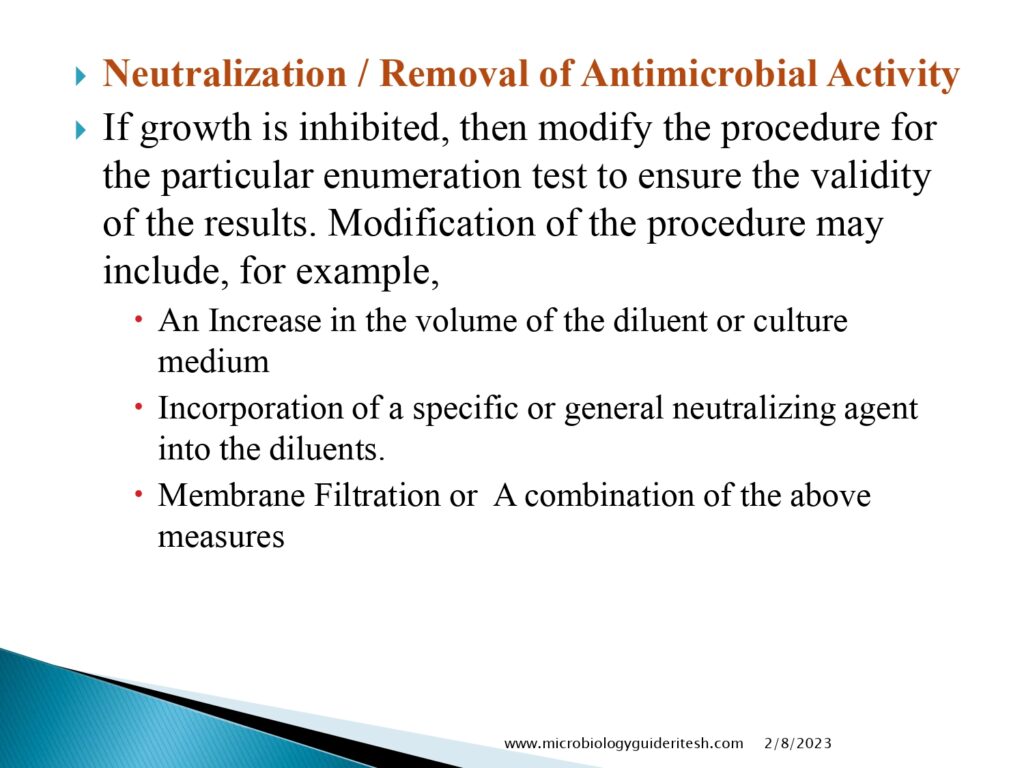
- Comparison with Acceptance Criteria: The obtained results are then compared with predefined acceptance criteria or standards. These criteria may vary depending on the specific regulations and guidelines applicable to the product being tested.
It’s important to note that the microbial limit test is a complex procedure that requires trained personnel and adherence to appropriate guidelines and regulations. It helps ensure that products meet the required quality and safety standards by providing information about the level of microbial contamination.
If you are specifically interested in a particular aspect or application of the microbial limit test, please provide more details so that I can provide more specific information.
Microbial levels must be controlled during the processing and handling of pharmaceutical or medical products or components. These products’ bio burden or microbial limit testing proves that these requirements have been met.

- Objective & Scope

- Responsibility:

- Accountability & Procedure



- Total Aerobic & Microbial Count Testing(Pour Plate Method):
- Total Yeast & mold Testing(Pour Plate Method):
- Specified Microorganism Test
FOR SPECIFIED MICROORGANISMS:
- For Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Clostridia
- Prepare the sample using 1 in 10 dilution of not less than 1 g of the sample to be examined as mentioned above (A) and use 10ml or the quantity corresponding to 1g or 1ml to inoculate in to suitable amount of Soyabean Casein Digest broth and mix.
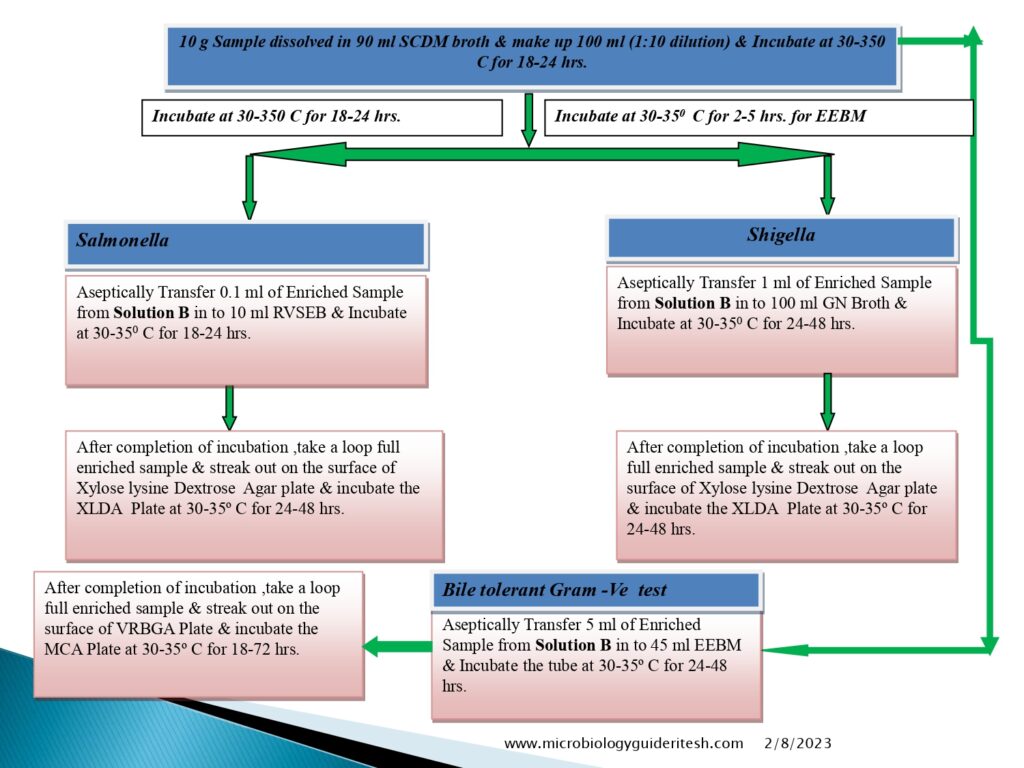
- For Salmonella, Shigella
- Prepare the sample to be examined as mentioned above (A) and use the quantity corresponding to not less than 10g or 10ml to suitable amount of Soyabean Casein Digest broth and mix.
- If limit mentioned as absent in 1g or 1ml sample then take the sample quantity 1 g or 1 ml and proceed further.
- For Bile-Tolerant Gram-Negative Bacteria (Enterobacteria):
- Prepare a sample using 1 in 10 dilution of not less than 1 g of the product to be examined as mentioned above (A) using soyabean casein digest broth as chosen diluent.
- For Candida albicans:
- Prepare the sample using 1 in 10 dilution of not less than 1 g or 1 ml of the sample to be examined as mentioned above (A) and use 10ml or the quantity corresponding to 1g or 1ml to inoculate in to suitable amount of Sabouraud Dextrose Broth and mix.

Flow diagram of Microbial Limit Test
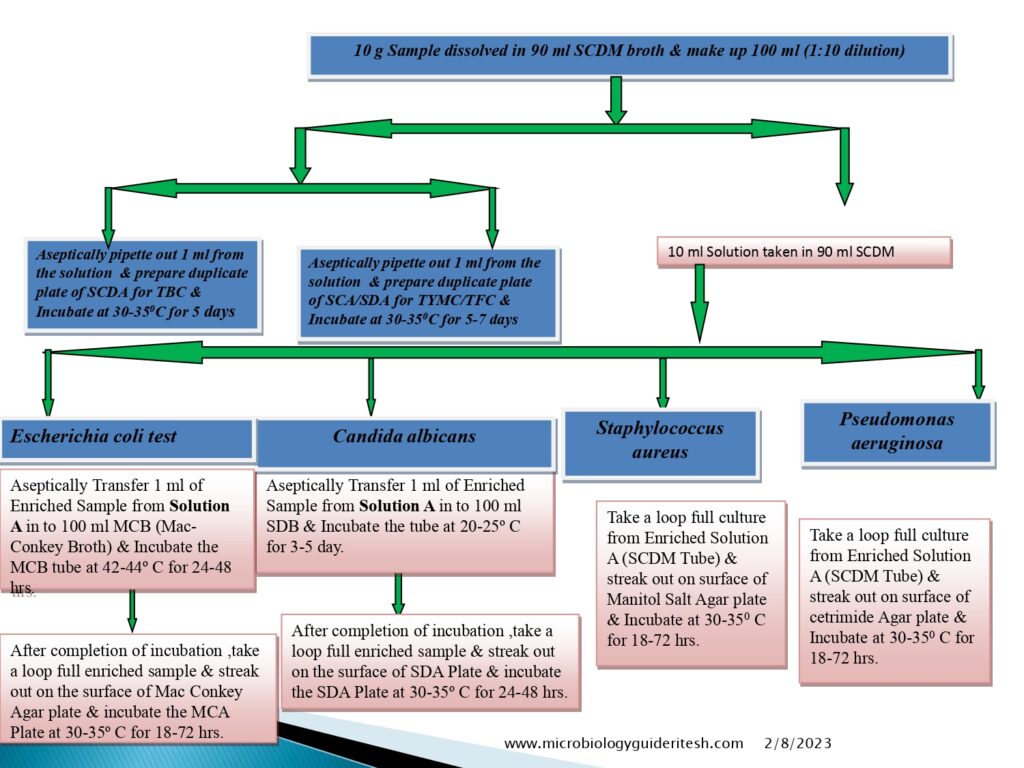
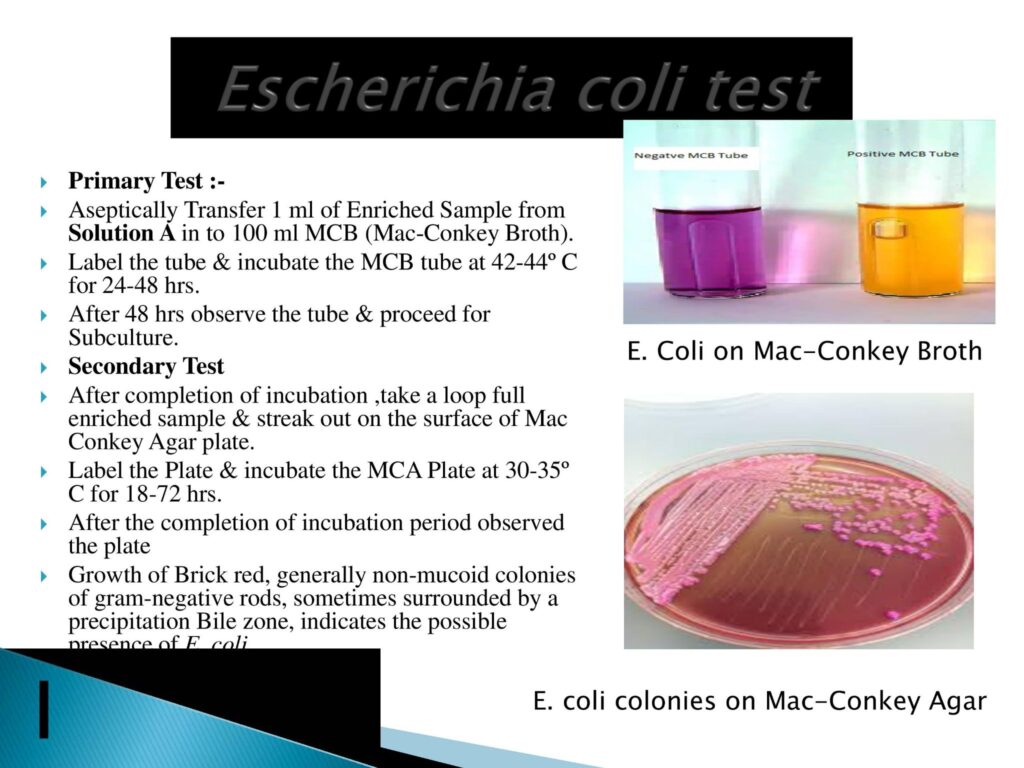
- E. coli Testing Method:-
- Escherichia coli is a gram-negative, facultative anaerobic, rod-shaped coliform bacterium of the genus Escherichia that is commonly found in the lower intestine of warm-blooded organisms. Most E. Coli strains are harmless, but some serotypes (epec, etec, etc.) can cause serious food poisoning in their hosts and are occasionally responsible for food/medicine contamination incidents that necessitate product recalls.
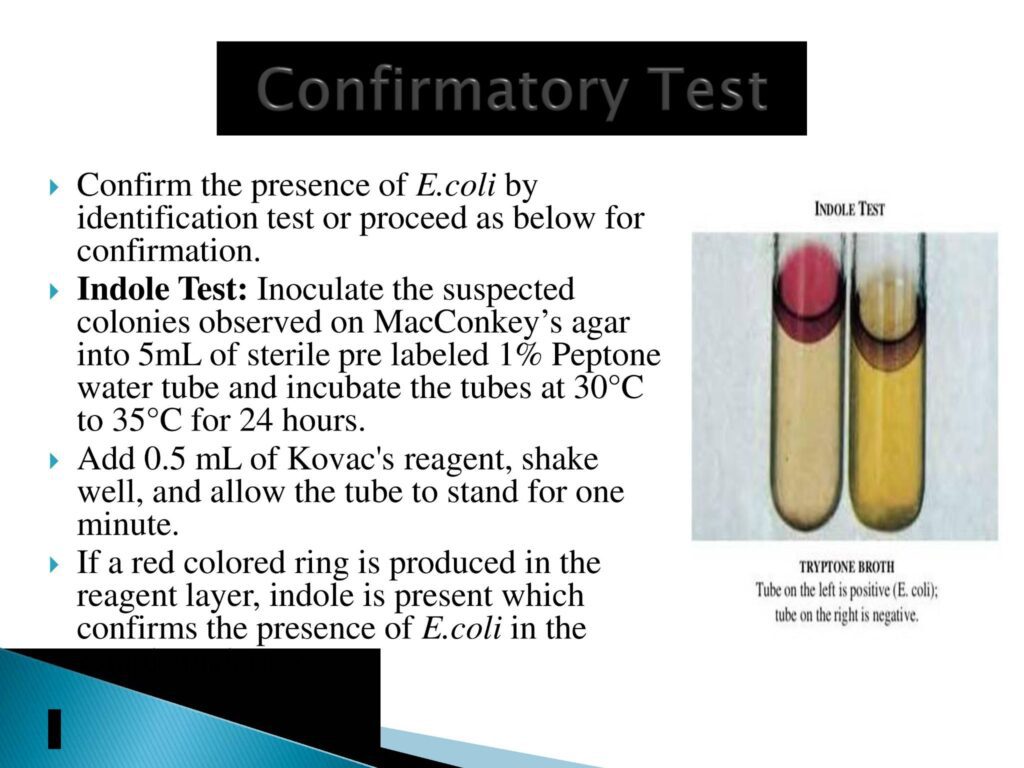
- Confirmatory Test:- Indole test
- Salmonella Test Method:-
- Salmonella enterica (formerly Salmonella choleraesuis) is a species of the genus Salmonella and a rod-headed, flagellate, facultative anaerobic, Gram-negative bacterium.
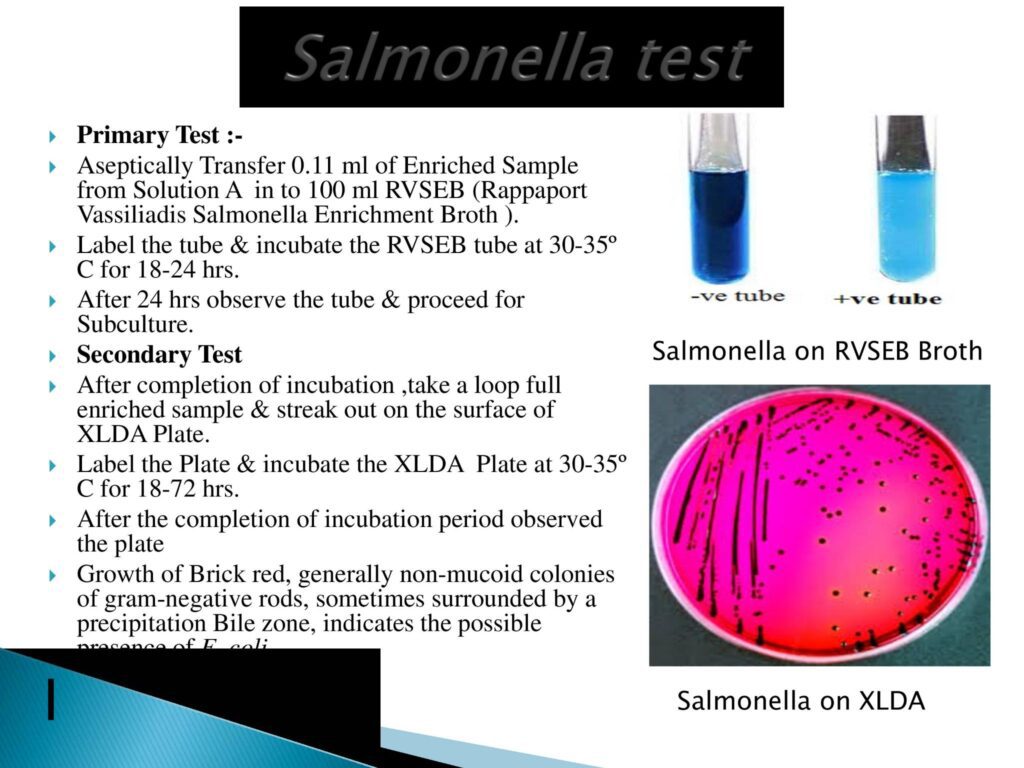
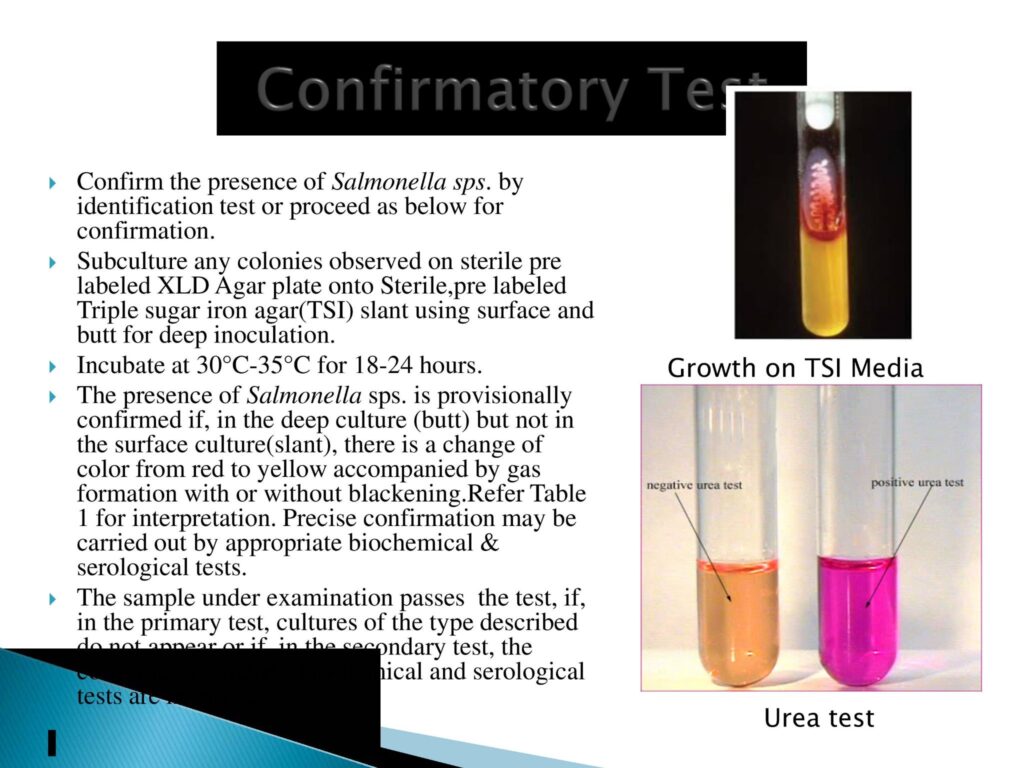
- Confirmatory Test:- TSI test & urease test
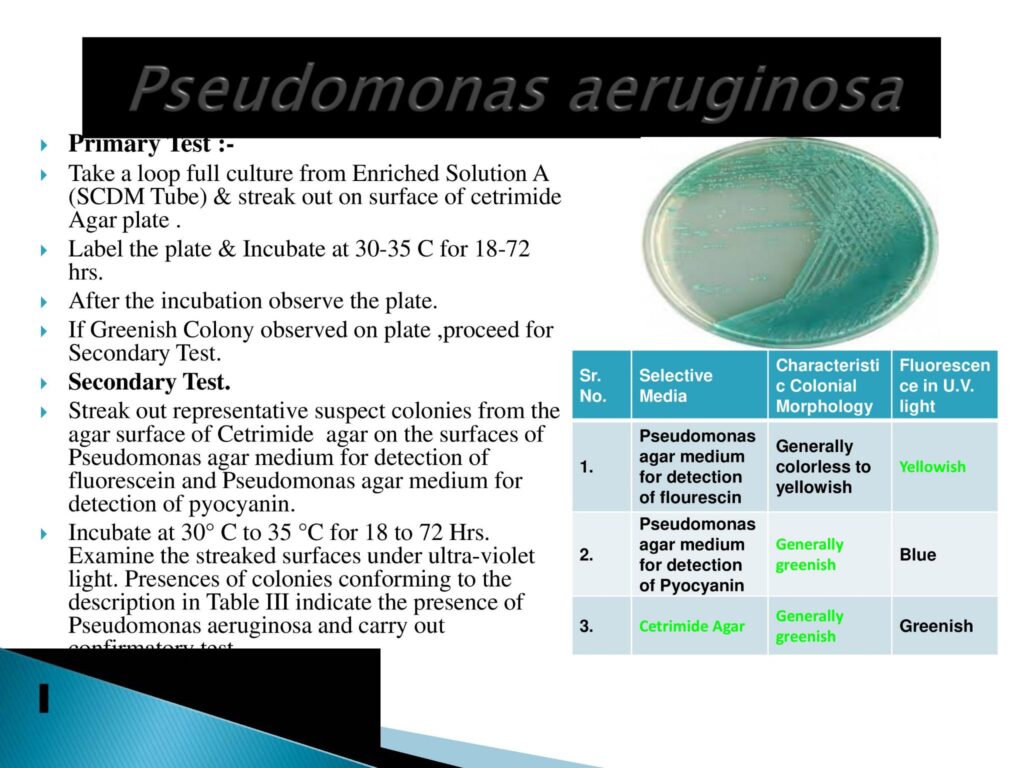
- Pseudomonas Test Method:
- Pseudomonas is a genus of Gram-negative bacteria that belongs to the family Pseudomonadaceae and the class Gammaproteobacteria.
- Rod-shaped
- Gram-negative
- Flagellumone or more, providing motility
- Aerobic
- Non-spore forming
- Catalase-positive
- Oxidase-positive
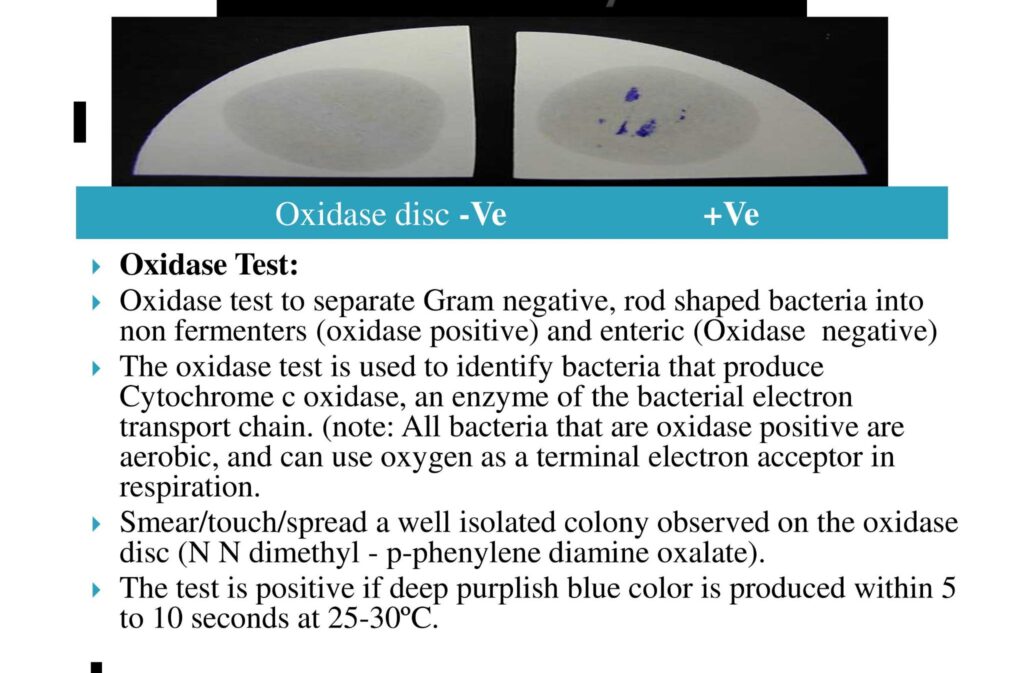
- Confirmatory Test:- Oxidase test
- Staphylococcus aureus Test Method:-
- Staphylococcus aureus is a Gram-positive spherically shaped bacterium that belongs to the Bacillota and is a common member of the body’s microbiota, commonly found in the upper respiratory tract and on the skin.

- Shigella Test Method:
- Shigella boydii is a Gram-negative bacterium of the genus Shigella. S. boydii, like other members of the genus, is a nonmotile, nonsporeforming, rod-shaped bacterium that can cause dysentery in humans via fecal-oral contamination.
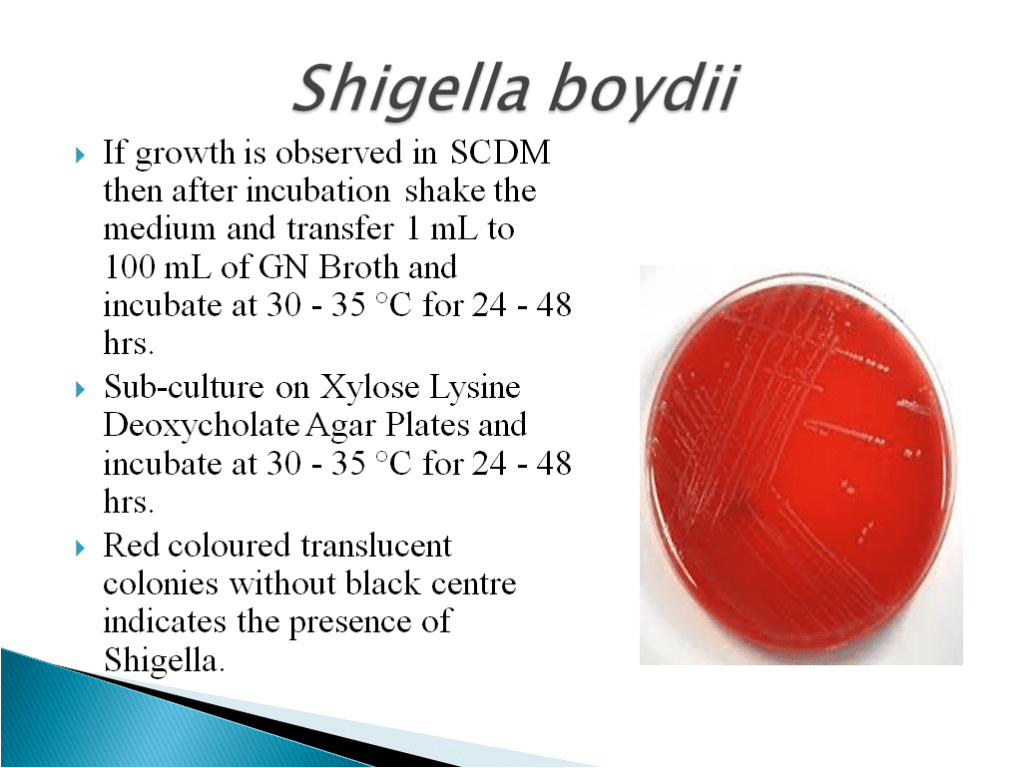
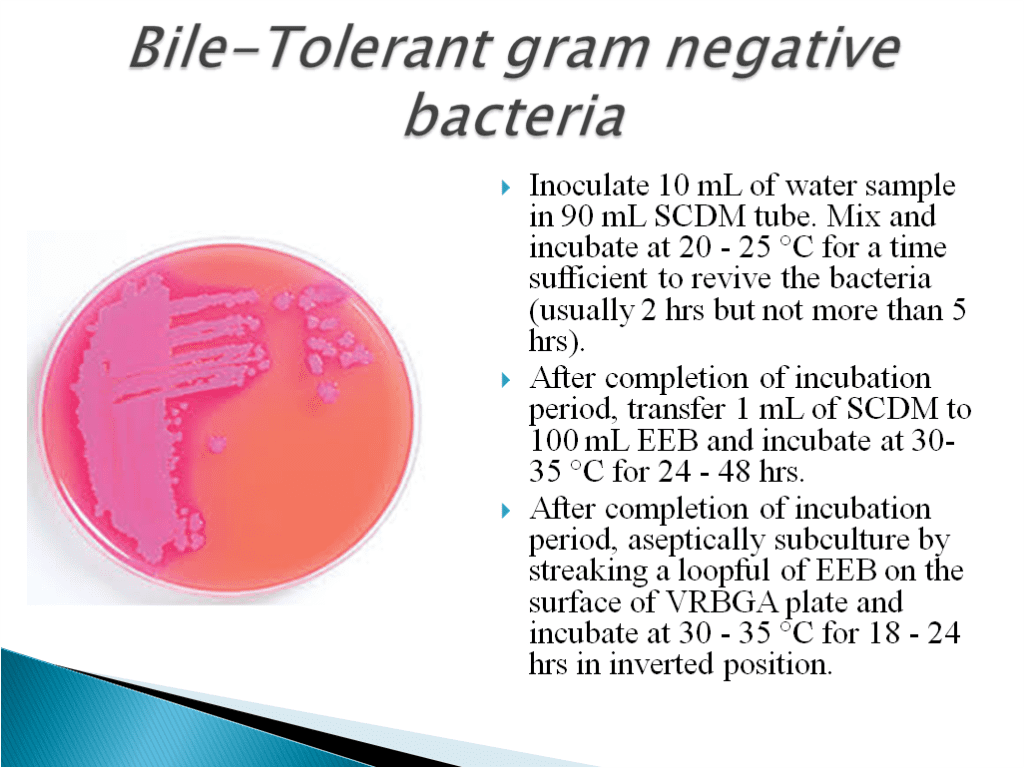
- Bile Tolerent Gram Negative Bacteria Test Method:
- Enterobacteriaceae is a large Gram-negative bacteria family. Rahn proposed it in 1936, and it now includes over 30 genera and over 100 species.
Its classification beyond the family level is still debated, but one classification places it in the order Enterobacterales of the class Gammaproteobacteria in the phylum Pseudomonadota.
Adeolu et al. (2016) revised the description and members of this family based on comparative genomic analyses. - Many of the more well-known pathogens, such as Salmonella, Escherichia coli, Klebsiella, and Shigella, are members of the Enterobacteriaceae family, along with many harmless symbionts. Enterobacter and Citrobacter are two other disease-causing bacteria in this family.
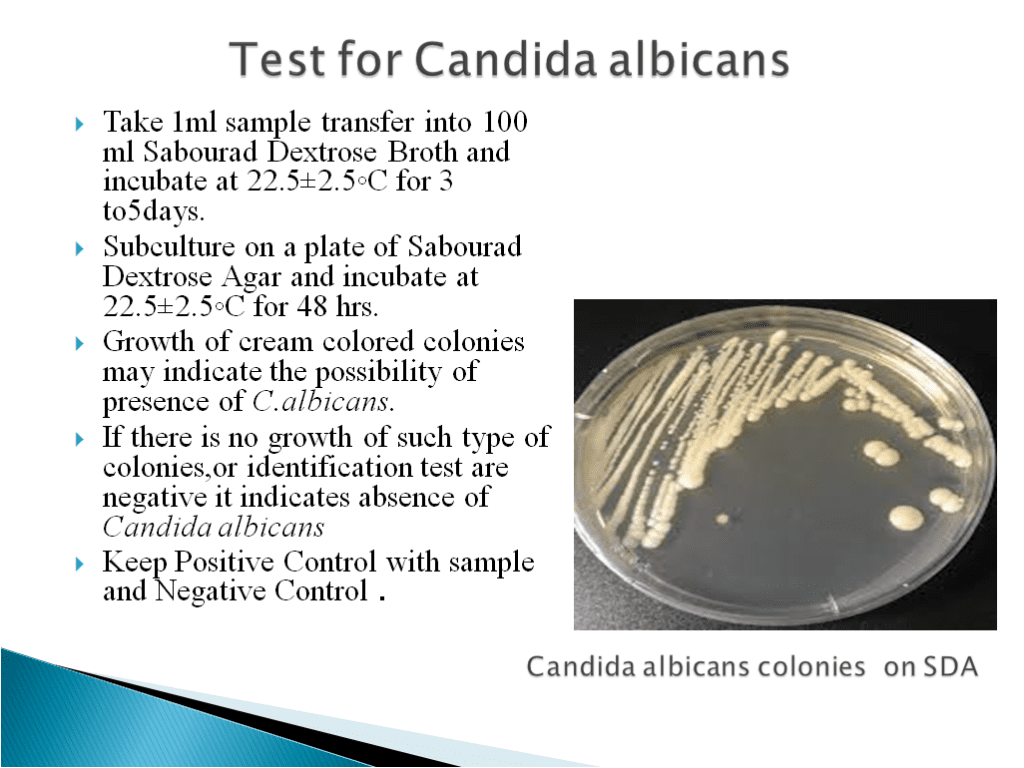
- Candida Test Method:-
- Candida albicans is an opportunistic pathogenic yeast that is a common member of the human gut flora. It can also live outside of the human body.
- It is found in the gastrointestinal tract and mouth of 40-60% of healthy adults.
- It is normally a commensal organism, but it can become pathogenic in immunocompromised individuals under a variety of conditions.
Pingback :-
- Microbiologist Qualification Quality 2023
-

Isolation & IIdentification

I’ve been absent for some time, but now I remember why I used to love this blog. Thanks, I will try and check back more often. How frequently you update your website?
This is a very good tips especially to those new to blogosphere, brief and accurate information… Thanks for sharing this one. A must read article.
Hello! I just would like to give a huge thumbs up for the great info you have here on this post. I will be coming back to your blog for more soon.
Its like you read my mind! You appear to know a lot about this, like you wrote the book in it or something. I think that you can do with some pics to drive the message home a little bit, but other than that, this is wonderful blog. A great read. I’ll definitely be back.
Greetings from Ohio! I’m bored to tears at work so I decided to browse your blog on my iphone during lunch break. I enjoy the information you provide here and can’t wait to take a look when I get home. I’m shocked at how quick your blog loaded on my mobile .. I’m not even using WIFI, just 3G .. Anyhow, awesome blog!