Definition of Environmental Monitoring:
Environmental monitoring refers to the systematic collection and analysis of data related to the environmental conditions within a specific area or facility. It is conducted to assess and manage potential risks to the environment, human health, and the quality and safety of products or processes. Environmental monitoring is performed in various industries, including pharmaceuticals, food production, healthcare, manufacturing, and research facilities.
Controlled area: An area in which specific environmental conditions are defined, controlled, and monitored to prevent contamination or degradation of the pharmaceutical starting material or product.
A controlled area is one in which unsterilized Drug products, in-process materials or containers/closures are prepared. Acceptable air quality if it has a per-cubic-foot particle count of not more than 100,000 in a size range of 0.5 microns and larger (Class 100,000) when measured in the vicinity of the exposed articles during periods of activity. (US FDA – Guidance for Industry Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice )
Clean room/Clean zone: An ISO-classified room in which the concentration of airborne particles is controlled to meet a specified airborne-particulate cleanliness class to prevent particle and microbial contamination and in which other relevant parameters e.g. Temperature, humidity and pressure are controlled as necessary.
Premises: Open (non build) area outside the manufacturing facility/building.
Environment Monitoring program: Documented program, implemented through standard operating procedures that describes in detail the procedures and methods used for monitoring. The program includes sampling site, frequency of sampling, and investigative and corrective actions that shall be followed if alert or action levels are exceeded. The methodology used for trend analysis is also described.
Environment Control: Steps taken through facility design and construction, testing and validation, personnel practices and cleaning and sanitization to limit micro-organisms in the clean room.
Environment Monitoring: Routine microbiological monitoring provides a series of snapshots of the microbiological profile of a controlled environment. Routine monitoring ensures that systems continue to provide an environment of consistent quality.
Biological safety cabinet (BSC): A ventilated cabinet with unidirectional HEPA-filtered airflow and HEPA-filtered exhaust to protect the worker from hazardous drugs. A BSC used to perform an activity must be capable of providing an ISO Class 5 environment for the activity in Environmental Monitoring .
Laminar airflow system (LAF): A device or zone within a buffer area that provides an ISO Class 5 or better environment for sterile compounding/activities. The system provides a unidirectional HEPA-filtered airflow.
Aseptic processing or preparation: A process by which separate, sterile components (e.g., drugs, containers, or closures) are brought together under conditions that maintain their sterility. The components can either be purchased as sterile or, when starting with non sterile components, can be separately sterilized prior to combining (e.g., by membrane filtration, autoclave) in Environmental Monitoring.
Alert Level/Limit: Indicates when a process might have drifted from normal operating conditions. An investigation may be performed and corrective action may be implemented, but no action is required. It can be assumed that repetitive excursions above the alert level may be addressed as if it were an action level.
Action Level/Limit: Indicates that a process has drifted from normal operating conditions. An investigation must be performed and corrective action must be implemented.
Specification Limit: It is the final limit for any test/parameter which is within the acceptable range defined by customer standards ,different guideline and/ or regulatory guideline to evaluate the quality in the process or product itself.
In-Operation/dynamic condition: The “in operation/dynamic” state is the condition where the installation is functioning in the defined operating mode and the specified number of personnel is present.
At rest condition: The “at rest” state is the condition where the installation is complete with equipment installed and operating in a manner agreed upon by the customer and supplier, but with no personnel present.
Sterile: Any material that has been subjected to the process of Sterilization (process that destroys or removes all viable microorganisms) is said to be sterile.
Any article that is free from living germs or microorganisms.
Non sterile: Any article that is not free from any living germs or microorganisms.
Critical area: Critical Areas (sites, zones, surfaces) are identified as those areas where sterilized product or container and/or closures are exposed to the environment
Critical sites, zones, surfaces shall be monitored most rigorously.
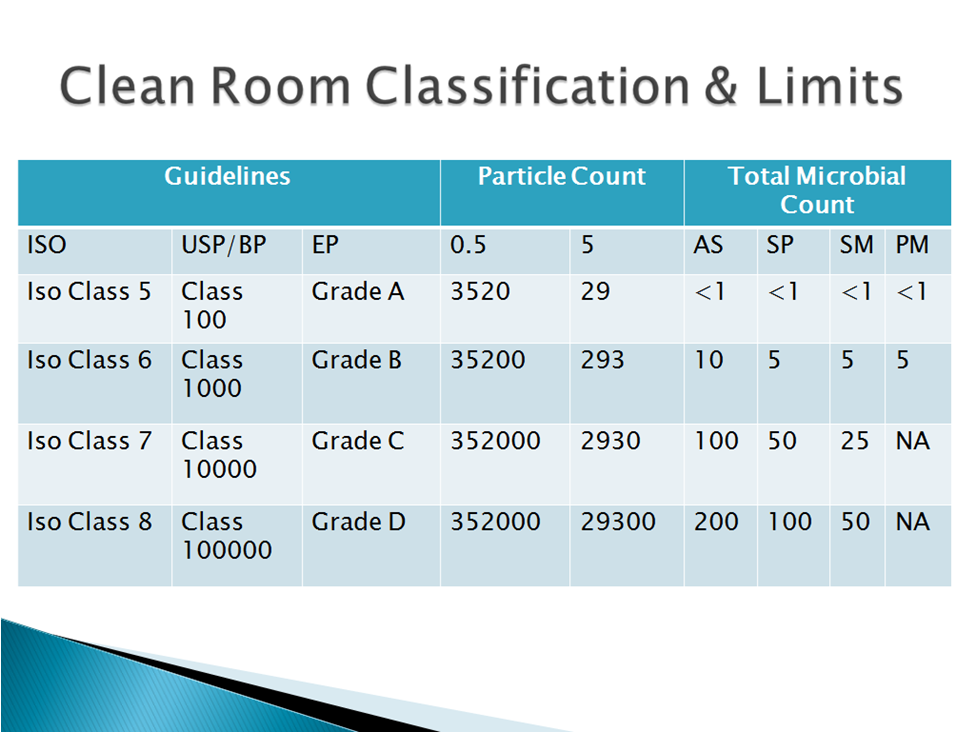
- Class 100 areas (EU Grade A).
- Organisms recovered from critical areas shall be identified to genus and species for possible investigation.
Grade A: The local zone for high risk operations, e.g. filling zone, stopper bowls, open Ampoules and vials, making aseptic connections. Normally such conditions are provided by a laminar air flow work station. Laminar air flow systems shall provide a homogeneous air speed in a range of 0.36 – 0.54 m/s (guidance value) at the working position in open clean room applications. The maintenance of linearity shall be demonstrated and validated. A unidirectional air flow and lower velocities may be used in closed isolators and glove boxes.
Grade B: For aseptic preparation and filling, this is the background environment for the grade A zone
Grade C and D (aseptic operations): Clean areas for carrying out less critical stages in the manufacture of sterile products
Grade D: Clean areas for manufacturing activities of various drug product/substances.
Labeling of Plates in Environmental Monitoring

Limits in Environmental Monitoring
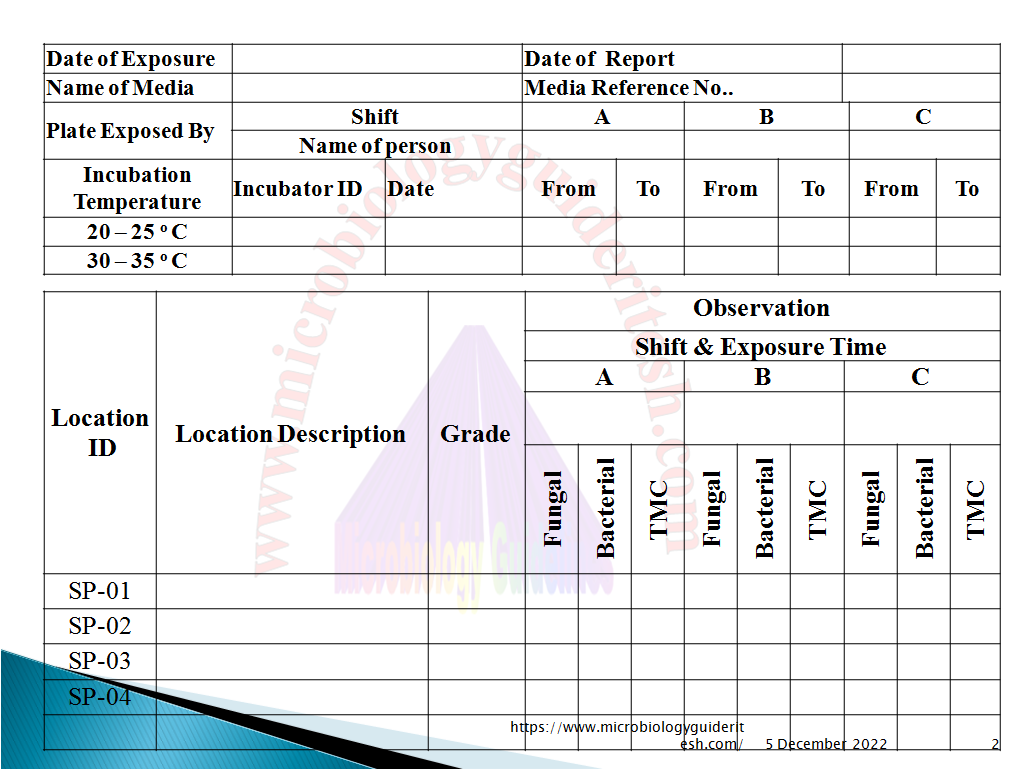
A. Settle Plate Exposure [Passive Air Sampling] in Environmental Monitoring:
- SCDA plates shall be prepared as per the procedure mentioned in the respective SOP.
- Pre-incubate the plates for 24 – 48 hrs at 30 – 35 °C.
- After the pre-incubation, the pre-incubated plates shall be examined for any microbial growth & if any growth has been observed discard the plate.
- Only fresh & without contaminated plates shall be use
- The personnel shall enter the Production/Store/Micro or respective Area as per respective SOP for Entry Exit Procedure.
- 6. The sampling kit shall be transferred to the respective area through the dynamic pass box.
- 7. Plates shall be exposed on plate exposure stand as per the locations mentioned in respective format for about 4 hours.
- Exposed SCDA plates shall be collected after about 4 hours of exposure and shall be incubated at 20 – 25 ºC for 72 hrs and further at 30 – 35 ºC for 48 hrs.
- Observe the plates after the completion of incubation period of the 5 days and record the observations in the respective format.
B. Active Air Sampling in Environmental Monitoring:
- Pre-incubated SCDA plates (90 mm) shall be used for the sampling of air at pre-defined location.
- 1000 liters [1 cubic meter] of air shall be sampled using the air sampler.
- Active air sampling plates shall be prepared as per the procedure mentioned in the respective SOP. The sieves shall be sanitized with 0.2 µ filtered 70 % IPA and shall be air dried before sampling.
- Visual check shall be done to verify any traces of IPA in the perforated sieve.
- Sequence of the air sampling shall be from critical zone to less critical zone.
- Perform air sampling by operating the Volumetric Air Sampler as per respective SOP.
- 1000 liters of air shall be sampled by the air sampler. After completion of air sampling, remove the sieve and aseptically place the cover lid on the plate, taking care not to touch the surface of media. Remove the plate from the air sampler.
- All the plates shall be recovered after sampling and shall be incubated at 20 – 25 ºC for 72 hrs for fungal growth and further at 30 – 35 ºC for 48 hrs for bacterial growth.
- Observe the plates after the completion of incubation period of the 5 days and record the observations in the respective format.
C. Surface Monitoring by RODAC in Environmental Monitoring:
- Pre-incubated contact plates always bring to aseptic area in SS container to avoid accidental or external contamination.
- Take the pre-incubated RODAC plates for surface monitoring.
- Carefully open the RODAC plates, invert and touch gently with convex surface of agar to the surface to be monitored.
- Press the plate firmly to expose the entire agar surface to the area to be sampled.
- Slowly take back the plate and close the plate with lid, take care not to leave any traces of agar medium to the surface monitored.
- Disinfect the sampled area and clean with sterile lint free cloth.
- Incubate the plates in inverted position in incubator at 20 – 25 °C for 72 hours and then at 30 – 35 ˚C for 48 hours.
- Observe the plates after the completion of incubation period of the 5 days and record the observations in the respective format in Environmental Monitoring .
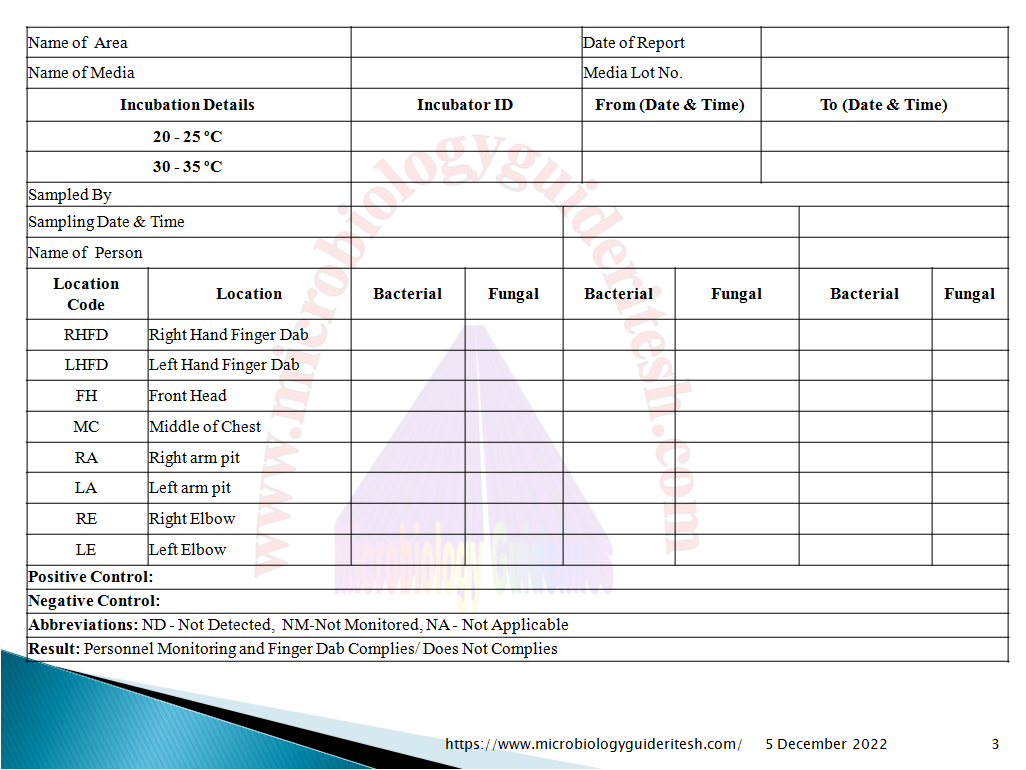
D. Personnel monitoring in Environmental Monitoring :
- Personnel monitoring by Gown monitoring shall be performed for all personnel’s working in the sterile manufacturing areas and Sterility testing area.
- The monitoring shall be performed during the exit of personnel from the area.
- Monitoring shall be performed for person working in Sterile (Aseptic) area and Sterility testing room.
- No monitoring surface shall not be sanitized just prior to monitoring.
- Plates (55 mm) of Soybean Casein digest agar with 1% glycerol (Wetting agent) and with or without Soya Lecithin and Tween 80 (neutralizing agents) or any specific neutralizer (e.g. Enzymes etc.) as per requirement shall be prepared in house or procured from an authentic source.
- The plates shall be pre- labeled with the Media name and date of preparation/expiry at a minimum.
- Person performing the gown monitoring can perform gown monitoring of his own gown or can perform gown monitoring of second person
- 8. Preferably monitoring of gown shall be done in presence of Microbiologist or QA.
- 9. The person monitoring the location shall open the RODAC plate lid aseptically and with gloved hands, then shall gently press the convex surface of the plate for 15-30 sec. on the location mentioned on the plates.
- Upon completion of contact the lid shall be placed back on the plate and the plates shall be placed in the sterile/sanitized container for transportation.
- Care shall be taken, while placing in the sterile/sanitized container, the plates of one person monitoring shall be kept together to avoid misplacing of various location monitoring plates
- At a minimum following locations of gown shall be monitored routinely in Environmental Monitoring .
-
-
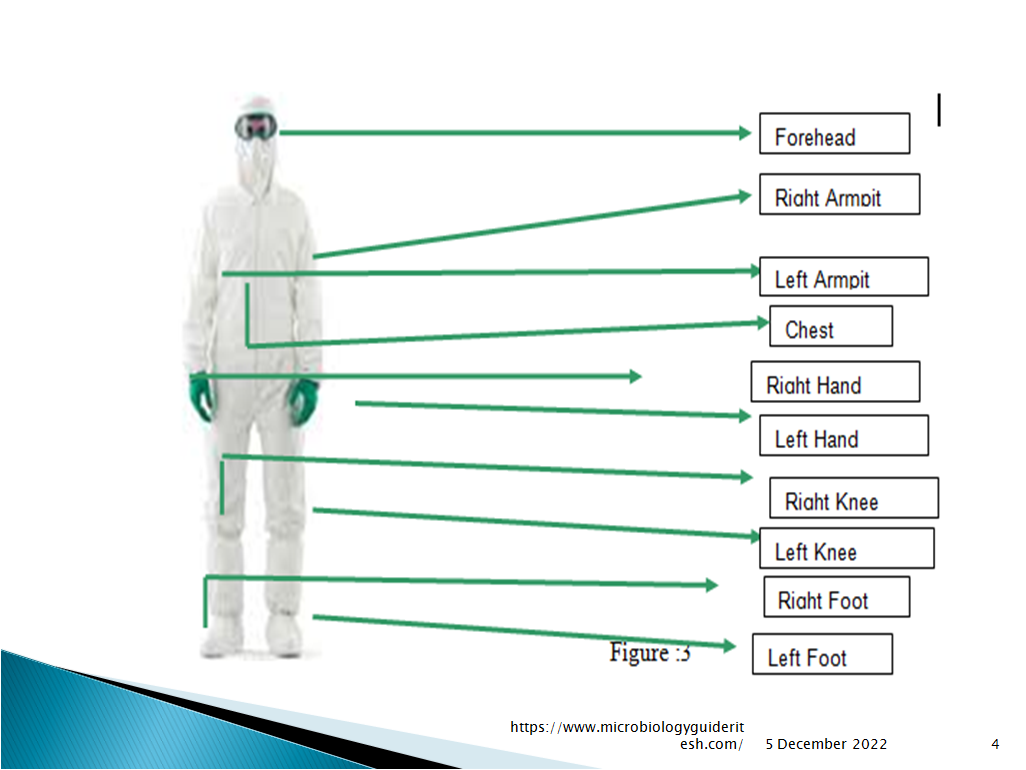
Forehead (FH)
-
Chest (CH)- Close to the zipper/Velcro
-
Right Armpit (RA)
-
Left Armpit (LA)
-
Left knee (LK)
-
Right knee (RK)
-
Left foot (LF)
-
Right foot (RF)
-
. Refer Fig: 3 for the various locations monitored on gown in Environmental Monitoring:
Annexure For Personal Monitoring in Environmental Monitoring
Annexure For Environmental Monitoring
You ma also read about. BET Bacterial Endotoxin Test

I’m usually to running a blog and i actually recognize your content. The article has really peaks my interest. I’m going to bookmark your website and preserve checking for brand new information.
An impressive share, I just given this onto a colleague who was doing a little analysis on this. And he in fact bought me breakfast because I found it for him.. smile. So let me reword that: Thnx for the treat! But yeah Thnkx for spending the time to discuss this, I feel strongly about it and love reading more on this topic. If possible, as you become expertise, would you mind updating your blog with more details? It is highly helpful for me. Big thumb up for this blog post!
Write more, thats all I have to say. Literally, it seems as though you relied on the video to make your point. You clearly know what youre talking about, why waste your intelligence on just posting videos to your weblog when you could be giving us something informative to read?