Microbiology Deviation:-
Microbiology deviation refers to a departure from established procedures or expected results.
Microbiology deviations can occur in various settings, including pharmaceutical manufacturing, food production, and research laboratories. These deviations may indicate potential issues with the quality, safety, or reliability of microbiological testing and processes.
Here are a few examples of microbiology deviations and their potential implications:
- Contamination events: Microbiological testing relies on aseptic techniques and sterile conditions to prevent contamination. If a deviation occurs, such as a breach in sterile technique or an equipment failure leading to contamination, it can result in false-positive or false-negative results. Contamination events can compromise the accuracy and reliability of microbiological testing, potentially leading to incorrect conclusions about the microbial quality of a product or environment.
- Out-of-specification results: Microbial limit testing involves comparing the obtained results with predefined acceptance criteria or specifications. If the test results exceed the established limits, it indicates a microbiology deviation. Out-of-specification results may suggest microbial contamination, inadequate sterilization procedures, or compromised product quality. These deviations may trigger investigations, corrective actions, and retesting to ensure compliance and prevent potential risks.
- Equipment malfunction or calibration issues: Microbiological testing often involves the use of specialized equipment, such as microbiological incubators, autoclaves, and colony counters. Deviations related to equipment malfunction or calibration errors can significantly impact the accuracy and reliability of test results. Regular maintenance, calibration, and validation of equipment are crucial to minimize the occurrence of such deviations.
- Procedural deviations: Any deviation from established procedures for sample collection, handling, preparation, or testing can introduce variability and potential errors in microbiological testing. Examples may include improper aseptic techniques, incomplete documentation, or deviations from the recommended incubation times or temperatures. These procedural deviations can compromise the integrity and reliability of the test results, leading to inaccurate assessments of microbial contamination levels or quality.
When microbiology deviations occur, it is important to investigate the root cause, document the findings, and take appropriate corrective actions. These actions may include retesting samples, implementing process improvements, revising procedures, or conducting training to prevent similar deviations in the future. Additionally, regulatory authorities, such as the FDA or relevant local agencies, should be promptly notified when deviations may impact product quality, safety, or compliance with regulatory requirements.
It’s worth noting that specific guidelines and protocols exist in different industries and organizations to address microbiology deviations. These guidelines often provide detailed instructions on how to handle and manage deviations to ensure the integrity and reliability of microbiological testing processes.
Deviation : A deviation is an activity performed differently and/or modified than that specified in an approved document.
Any deviation occurred in breakdown or manual error shall be termed as deviation.
1.0 OBJECTIVE:
To provide a procedure to be followed in case of Microbiological Data Deviation from the normal.
2.0 SCOPE:
2.1 The procedure is applicable to all Microbiological testing methods followed at Microbiology Lab
2.2 The procedure is applicable for all investigation & evaluation of Microbiological Data Deviation such as Alert/Action limit excursion, Out of Specification (OOS) in Microbiological analytical results / observation in Microbiology Labs (Microbial Enumeration test, Test for specified microorganisms, Sterility test, water quality monitoring , Environmental monitoring and BET testing).
3.0 RESPONSIBILITY:
3.1 Microbiologists/Trained personnel
3.1.1 To inform the data deviation in any specific test immediately.
3.1.2 To participate in the investigational process.
3.1.3 To perform the activities as per procedure.
3.1.4 To report any non compliance to the procedure.
3.2 Head-Microbiology
3.2.1 To ensure the activities are performed as per procedure
3.2.2 To initiate the process of investigation and complete the same as per procedure.
3.2.3 To ensure the activities are performed as per the laid down schedules.
4.0 ACCOUNTABILITY :
4.1 Head Quality Control/Designee (Location)
4.2 Head Quality Assurance/Designee (Location)
5.0 PROCEDURE:
5.1 Definition:
- Aseptic technique: Aseptic technique refers to the procedure used to avoid the introduction of microorganisms into the vulnerable site. The principle aim of an aseptic technique is to protect the patient/articles/materials from contamination by pathogenic organisms.
- Environment Monitoring programme: Documented program, implemented through standard operating procedures that describes in detail the procedures and methods used for monitoring. The program includes sampling site, frequency of sampling, and investigative and corrective actions that shall be followed if alert or action levels are exceeded. The methodology used for trend analysis is also described.
- Environment Monitoring: Routine microbiological monitoring provides a series of snapshots of the microbiological profile of a controlled environment. Routine monitoring ensures that systems continue to provide an environment of consistent quality.
- Alert Level/Limit: Indicates when a process might have drifted from normal operating conditions. An investigation may be performed and corrective action may be implemented, but no action is required. It can be assumed that repetitive excursions above the alert level may be addressed as if it were an action level.
- Action Level/Limit: Indicates that a process has drifted from normal operating conditions. An investigation must be performed and corrective action must be implemented.
- Specification Limit: It is the final limit for any test/parameter which is within the acceptable range defined by customer standards ,different guideline and/ or regulatory guideline to evaluate the quality in the process or product itself.
- Laminar airflow system (LAF): A device or zone within a buffer area that provides an ISO Class 5 or better environment for sterile compounding/activities. The system provides a unidirectional HEPA-filtered airflow.
- CAPA-Corrective and Preventive action:A reactive measure taken to eliminate the cause of an incident in order to avoid recurrence, or a proactive measure taken to prevent the cause of a potential incident from occurring.
- Repeat analysis:Repeat of a test using new sample (re-sample) carried out be two analyst one the analyst who had carried out the test initially and the second analyst/microbiologist with a expertise in the test.
- Out of Specification (OOS): Any microbiological result that does not comply with the specification limits.
- Failure: The condition or fact of not achieving expected result; a cessation of proper functioning or performance.
- Preliminary Laboratory Investigation Report (PLIR): It is a document of initial phase of investigation of deviation. It consists of step by step investigation done in order to determine whether the results are due to laboratory error or equipment/instrument malfunctioning.
5.2 Identifying and assessing of Microbiological Data Deviation:
- Analyst is responsible to perform analysis as per standard procedure and / or pharmacopoeia, in case of any excursion from the laid down limits (Alert/Action limit) and excursion from Specification limit (OOS result) is noticed by the analyst during analysis, analyst shall immediately inform to Head Microbiology/Quality Control or his Designee.
- Head – Microbiology or Designee shall review the data as per standard operating procedure and discuss with Analyst regarding the entire procedure followed for analysis.
- If the excursion is from the Alert /Action limit in Environment Monitoring and Water quality Monitoring samples then an intimation of the excursion shall be given through appropriate SOP on Environment Monitoring and Water quality Monitoring.
- Analyst shall preserve all media, plates, test tubes, culture dilutions including all glassware (which are available on the day of the observation for excursion) used for the investigation and identification purposes.
- If after discussion and verifying all criteria/data, if it is found that the analytical results are out of specification, the laboratory investigation shall be carried out.
- Analyst shall preserve all standard and sample preparation solution, media, plates, test tubes, culture dilutions including all glassware (which are available on the day of the deviation/OOS observation) used for the investigation and identification purposes.
- The test solution, media plates, test tubes, culture dilutions etc. shall be transferred to temperature controlled chamber maintained at 2-8ºC till the investigation is completed and a disposition decision is taken by Head QC/Head QA.
- If the samples, test solution, media plates, test tubes etc. are not available, recorded data shall be reviewed.
- The analyst shall get the OOS Laboratory Investigation Form from QA and fill the details.
- Authorized QA person shall allocate the unique alphanumeric identification numbering of OOS as mentioned below:
MB/OOS/22/XXX/0001
Where:
LLL : Location Code
MBL : Microbiology Lab
OOS :Out of Specification
22 : Last two digits for Year of OOS observation
XXX : Name of the test
0001 : Serially numbered OOS No.
12. Code of Test name given below in table:
Name of test Code
Microbial Enumeration Test-MET
Water Sample Tests-WAT
Environment monitoring-EM
Personnel monitoring-PM
Bacterial Endotoxin Test-BET
Sterility Test-STE
Microbial Assay of Antibiotics-MAA
Test for specified microorganisms-TSM
Microbial Assay of Vitamins-MAV
Method Suitability test -MST
Preservative efficacy test-PET
- Authorized QA person shall provide the OOS number and enter the initial details of the OOS in the OOS Laboratory Investigation Form
- The OOS Investigation form after entering initial details shall be given to Head QC /Head Microbiology/Designee for further investigation.
- The investigation shall be thorough, timely, unbiased, well documented and scientifically defensible. Investigation shall be justified by an assignable root cause.
- For reference use Annexure I (Flow chart of OOS for Intermediate/Finished Product Analysis) & Annexure II (Flow chart of OOS for Microbiological Water sample analysis, Personnel Monitoring & Environmental Monitoring results in Microbial testing parameters).
- There is no need for a formal laboratory investigation in situations like instrument/equipment failure, power failure, in-complete transfer of a sample which are identified immediately, spillage of sample solution, etc. Such cases shall be handled through SOP on Laboratory incident handling
- If errors are obvious, such as the spillage of a sample solution, increase in the temperature of equipment is identified or the incomplete transfer of the sample is observed, the analyst should immediately document the same and put remarks for invalidation on the analytical raw data and take necessary action. Analysts should not knowingly continue an analysis they expect to invalidate at a later time for an assignable cause. It shall be handled through SOP of Laboratory incident handling if analyst observed event online before obtaining of results.
- If multiple OOS are observed in Environment and Water Samples for a day then one OOS No. shall be allotted for a day for one area/water system and all the various observations for various tests shall be included in one OOS.
- If consecutive sampling show out of specification results, the monitoring shall be stopped in consultation with QA and shall be resumed only after appropriate measures are taken to reduce the microbial load in the area/water system
- If a person show a high count in the consecutive sampling days in personnel monitoring, then the person shall not be allowed to work in the aseptic areas and counseling and training of the personnel shall be conducted.
- Upon satisfactory personnel re qualification the person shall be allowed to enter in the aseptic manufacturing area. The details shall be documented appropriately.
- If the excursion/OOS is of a repeated nature for a location or observed on consecutive days then all the excursion and OOS shall be investigated through one Excursion no./OOS ID and no separate excursion/OOS nos. shall be generated.
- If multiple OOS are observed for a location/water system and no corrective action is effective then an increased sanitization of the area/system shall be carried out and if required the frequency of sanitization shall be revised, care shall also be taken to reduce/restrict handling of sample. If required, check for any change in the sanitizing agents based on the isolated organisms shall also be done.
- Under such circumstances Head QA in consultation with Corporate Quality shall take a decision on the manufacturing activities in the area.
- If required an appropriate risk assessment shall be prepared to evaluate the impact of the excursions/OOS on the quality of product/s.
5.3 Microbiological Data deviation during Media Growth Promotion:
- Following data deviation can be observed during media growth promotion:
- The investigation shall be carried out as per CSOP on Procedure for Media Management CSOP/2014/015 and the formats mentioned in the CSOP shall be taken for investigation.
- Growth observed in negative control tubes/plates or in plates for the Test of Inhibition:
- The reason could be analyst error during handling/preparation of the media.
- Identify the particular colonies and study the characteristics.
- If colony observed is one of the isolates, check the whole lot of media tubes or plates.
- If many of the media containers are showing growth without inoculation check whether the steam sterilizer is in calibrated state during sterilization of media.
- Check for proper functioning of steam sterilizer. If required, re-validate the instrument.
- Dispose off the entire lot of media and proceed for investigation as per CSOP on Procedure for Media Management and the formats mentioned in the SOP shall be taken for investigation.
- Thoroughly evaluate the samples analyzed using that particular media.
- If any failure is observed and the media has been used for any sample analysis before GPT release, disregard the analysis and re analysis shall be carried out of those samples using fresh and approved lot of media.
- Growth is not detected/accepted recovery is not obtained in Positive control/Indicative test:
- Check for calculation error, if any.
- Check whether culture inoculum dilution added is valid for used
- If culture is valid, whether inoculum procedure followed is correct.
- Check for incubation conditions are appropriated.
- Check for media should not be over heated during sterilization or heating or re melting.
- Check for whether steam sterilizer/incubator is in calibrated state during sterilization/incubation of media.
- Check for proper functioning of instrument/equipment, if required, re validate the instrument/equipment.
- Dispose off the entire lot of media and proceed for investigation as per SOP on Procedure for Media Management .
- Thoroughly evaluate the samples analyzed using that particular media.
- If any failure is observed and the media has been used for any sample analysis before GPT release, disregard the analysis and re analysis shall be carried out of those samples using fresh and approved lot of media.
5.4 Microbiological Data deviation during Pure culture maintenance:
- Growth not observed during plating for inoculum preparation:
- Check for the viability/expiry of the culture.
- Check for the proper handling techniques used.
- Check for appropriate incubation/storage conditions.
- Investigate the reason for the incidence.
- Revert to stock culture passage tube for fresh inoculum preparation.
- Check for calibration status and proper functioning of Incubator and cold chamber. If required re validate the instrument.
- If growth is not observed further then procure fresh culture and proceed as per SOP on Microbial Culture maintenance and enumeration.
5.4.2 Contamination observed in pure culture plates/slants:
- 5.4.2.1 Check for the proper handling techniques during inoculum preparation.
- 5.4.2.2 Identify the particulars of the contaminant colony.
- 5.4.2.3 Investigate the reason for the incidence.
- 5.4.2.4 Revert to stock culture passage tubes for fresh inoculum preparation.
- 5.4.2.5 If pure culture is still not obtained then procure fresh culture and proceed as per CSOP on Microbial Culture maintenance and enumeration.
5.5 Microbiological Data deviation during testing of pre sterilized items and diagnostic kits used in Microbiology Lab:
- If results are unusual then the materials shall be rejected and sent back to the manufacturer immediately.
- The material shall not be used for any further testing.
5.6 Causes of OOS Test Result
- Assignable cause: An assignable cause is an identifiable, specific cause of variation in an analysis or measurement. A cause of variation that is not random and does not occur by chance is “assignable”
- If assignable cause is found then the analysis shall be considered void.
- Some of the common Laboratory error during analysis are as follows, but not limited to:,
5.6.3.1 Error in sampling, handling or storage of sample
- Error in following the method of analysis: Incorrect method used, wrong instrument used, parameters not correctly used.
- Error in usage of media and culture suspensions.
- Error during sterilization of media and accessories used in testing.
- Error in cleaning and sanitization of testing facility.
- Error during observation.
- Error in calculation/ typographical mistakes.
- Due to instrument/equipment malfunction identified at the time of OOS
- Use of non calibrated instruments & / or apparatus.
- Use of invalid / incorrect working standard due to mishandling of working standard or use of wrong working standard
- Use of contaminated glass wares and apparatus.
- Lack of training / knowledge of analyst
- Use of expired or old chemicals, reagents, medias, cultures or solvents.
- Improper storage and handling of samples.
- Error in sampling procedure
- Inadequate transfer of sample / solution / Wrong weighing /due to analytical error like dilution/filtration/sonication or extraction etc.
- Software error due to software malfunctioning
- Cross contamination during analysis
- Others if any.
- Non – assignable cause: In such cases where no assignable root cause identified,, additional data must be generated to assess whether the initial a typical result is a statistical outlier.
5.7 Excursion in Microbial Counts (Air/Surface/Personnel/Drain Monitoring) beyond Alert Limit-
- When the alert limit is crossed then intimate the Department Head / Manufacturing Head / QA Head as per SOP on Environment Monitoring and format mentioned in the SOP.
- When microbial counts exceed the alert limit increase the cleaning and sanitization in the area with double frequency till counts come to normal.
- If Microbial counts are beyond alert limit for 3 consecutive days then the same shall be considered as an excursion beyond action limit and the measures shall be taken up according to the action limit excursion.
- Monitoring of the area with routine frequency can provide assurance for the area status.
5.8 Excursion in Microbial Counts of Air Monitoring beyond Action Limit-
- When the alert limit is crossed then intimate the Department Head / Manufacturing Head / QA Head as per SOP on Environment Monitoring and format mentioned in the SOP.
- When microbial counts of area (Settle plate exposure or Air sampling) exceeds the action limit, follow the action plan as given below:
- Check manometer reading and particle count of area.
- If above tests are found within specified limit, increase the cleaning and sanitization in the area with double frequency.
- Increase frequency of microbial monitoring to twice in a day for three days for Sterile Manufacturing areas /Sterility Testing area.
- Perform microbial monitoring for three days for Non Sterile Manufacturing areas /other testing areas of Microbiology Lab.
- If any of the microbial monitoring results is not found within specified limit, stop the activities (Manufacturing/Filling/Testing) in the area.
- Engineering department shall be called for the corrective action as per applicable site specific SOPs.
- Investigate the reason for the excursion as per SOP on Environment Monitoring and format mentioned in the SOP.
- The investigation shall include the following but not limited to
-
- Review level of personnel activity in the area on that day
- Review/perform airflow patterns and HEPA integrity tests results during last qualification
- Review aseptic technique of personnel and training records
- Review gowning procedures and other requirements for area
- Review trends and any incidents of HVAC outages, if they occurred
- Inspect incoming air filters for leaks and pressure differential across filter
- Review room disinfection and sanitization procedures, sanitization intervals and disinfectant efficacy.
- Review training records of individuals performing sanitization or disinfection
- Check area pressure differentials, particularly with respect to the last sanitization
- Evaluate mechanical equipment in the area as possible source of contamination
- Review relevant, recent data at the same sites and subsequent monitoring results available.
- Review sterilization cycle documentation and records.
- After corrective action perform the microbial monitoring of the area with increased frequency of twice in a day for three consecutive days for Sterile Manufacturing areas /Sterility Testing area.
- Perform microbial monitoring for three days for Non Sterile Manufacturing areas /other testing areas of Microbiology Lab. .
5.9 Excursion in Microbial Counts of Surface/Drain Monitoring beyond Action Limit-
- When the alert limit is crossed then intimate the Department Head / Manufacturing Head / QA Head as per SOP on Environment Monitoring and format mentioned in the SOP.
- In case of microbial count of surfaces exceed the action limit:
- Check that the disinfectant used is as specified for surface sanitization.
- Increase the cleaning and sanitization with double frequency.
- Train the personnel for clean room practices
- Increase frequency of surface monitoring to twice in a day for three days for Sterile Manufacturing areas /Sterility Testing area.
- Perform surface monitoring for three days for Non Sterile Manufacturing areas /other testing areas of Microbiology Lab.
- If any of the surface monitoring results is not found within specified limit, stop the activities (Manufacturing/Filling/Testing) in the area.
- Engineering department shall be called for the corrective action as per site specific SOPs.
- Investigate the reason for the excursion as per SOP on Environment Monitoring and format mentioned in the SOP.
- The investigation shall include the following but not limited to
Perform investigation for possible sources of contamination.
Evaluate sanitization and disinfection practices, review preparation of disinfectants, cleaning records, and training records of individuals performing sanitization and disinfection.
-
-
-
Review possible unusual events during manufacturing /testing operation
-
Examine areas during operation for the day of further monitoring to under stand behavior and practices of personnel
-
Review closed circuit video (if applicable)
-
Verify that controls w
-
rence in other types of tests
-
Evaluate integrity of the room (e.g. Peeling of paints or cracks in ceiling, walls and floor)
-
Examine endotoxin and water chemical test data for the system
-
Review for the excursion in water samples used for cleaning and disinfection purposes.
-
ere not circumvented
-
Review risk of product contact
-
Review isolates for occur
-
-
- After corrective action perform the surface monitoring of the area with increased frequency of twice in a day for three consecutive days for Sterile Manufacturing areas /Sterility Testing area.
- Perform surface monitoring for three days for Non Sterile Manufacturing areas /other testing areas of Microbiology Lab. .
5.10 Excursion in Microbial Counts of Personnel Monitoring beyond Action Limit-
- When the alert limit is crossed then intimate the Department Head / Manufacturing Head / QA Head as per SOP on Environment Monitoring and format mentioned in the SOP.
- Investigate the reason for the excursion as per SOP on Environment Monitoring and format mentioned in the SOP.
- The investigation shall include the following but not limited to
Evaluate possible operator impact on product
-
-
-
Review current environmental monitoring data and sterility test data
-
Review preparation and expiry dates for disinfectants used on gloves
-
Identify all morphologically unique isolates(human vs environmental)
-
Interview operator for potential cause and retrain or re qualify operator.
-
Evaluate training of operator
-
Review sanitization and disinfection records of area
-
Review closed circuit video (if applicable)
-
Review previous gowning data for the operator and other operators on the same day.
-
-
- In case of microbial count of finger dabs/gown monitoring of personnel exceed the action limit:
- Train the personnel for clean room practices.
- Increase the frequency of personnel monitoring to twice in a day for three consecutive days for Sterile Manufacturing areas /Sterility Testing area.
- If still the counts of a specific personnel are showing excursion, the person shall not be allowed to perform any aseptic handling in the area and shall be only allowed after complete personnel qualification as employed for an initial personnel qualification of a personnel.
5.11 Excursion in Microbial Counts for Water Samples beyond Alert Limit-
- When the alert limit is crossed then intimate the D.M. Water Plant Head / Manufacturing Head / QA Head / Unit Head as per SOP on Water quality Monitoring and format mentioned in the SOP.
- When microbial counts exceed the alert limit increase the cleaning and sanitization of the water system with double frequency till counts come to normal.
- If Microbial counts are beyond alert limit for 3 consecutive days then the same shall be considered as an excursion beyond action limit and the measures shall be taken up according to the action limit excursion.
- Monitoring of the water system with routine frequency can be continued to provide assurance of the system.
5.12 Excursion in Microbial Counts for Water Samples beyond Action Limit-
- When the action limit is crossed then intimate the D.M. Water Plant Head / Manufacturing Head / QA Head / Unit Head as per SOP on Water quality Monitoring and format mentioned in the SOP.
- When microbial counts exceeds the action limit, follow the action plan as given below:
- When the action limit is crossed then plant shall be sanitized immediately. Sampling and testing shall be done on the consecutive day of the information only after sanitization activity.
- Whenever action limit is crossed identify the organism based on its morphological characteristics.
- In case, there are continuous alert /action level excursion and colony morphology is same as identified then identification can be performed only colonies with different morphology not identified earlier.
- Increase frequency of microbial monitoring to twice in a day for three days if for 3 consecutive days excursion above action limit is observed. The test shall be performed for the specific test parameter only for the specific location.
- If any of the microbial monitoring results is not found within specified limit, stop the activities (Manufacturing/Filling/Testing) in the area.
- Engineering department shall be called for the corrective action.
- After corrective action perform the microbial monitoring of the system with increased frequency of twice in a day for three consecutive days.
5.13 Laboratory Investigation-Phase I (Primary Laboratory Investigation) for OOS in any of the parameter:
- Interrogate the analyst/operator for all details of procedure he/she followed, which will give an idea of his/her understanding of the procedure and the probable cause, if any, of the OOS.
- Laboratory Investigation (Primary Laboratory Investigation) shall be performed for all the OOS results / observations. First phase of investigation should include as initial assessment of the accuracy of the laboratory’s data. Whenever possible, this should be done before test preparation is discarded.
- Laboratory Investigation shall include but not limited to :-
- All the steps covered in point 5.3.
- Perform the isolation and identification of contamination from the OOS sample.
- Identification of contaminant micro-organisms shall be performed up to species level if feasible as per CSOP on Microbial Identification and maintenance of culture library.
- Perform the identification of colonies observed with new morphology from water, environment, and personnel monitoring samples during the period of sampling, analysis and incubation of OOS sample.
- Check the samples analyzed on same day, using same or different lot of media, incubated in the same incubator, using the same stock of culture concentrations for the positive control and growth promotion test.
- Check the weight of media, pH of media, sterilization cycles of the media and glass wares, storage of the sterilized media and glass wares.
- Check the training and certification status of microbiologist.
- Check the glassware management (Washing, sterilization and storage) used for the testing purpose.
- Check media receipt, qualification, usage, preparation, sterilization, storage & hold time limits against the procedure/validations.
- Check the cleaning and sanitization status of Analytical / working area (media preparation area, analytical area, incubation area, LAF etc.)
- Correlate the isolated contaminant with micro-organisms in the culture library.
- Check equipment / instrument Qualification, Validation, calibration and cleaning status.
- Check environment monitoring record (temperature, humidity, pressure differential, settle plate count, active air sampling count, surface monitoring, particle count and personnel monitoring).
- Check for any power failure during sampling, analysis, sterilization and incubation. Check for any abnormal observation during sampling, analysis, sterilization and incubation.
- Check status of positive controls, negative controls & product positive controls.
- Check results / observations of other analysis performed on same day by same microbiologist for sample under question and also check for the observation / results of test performed using same area by other microbiologist.
- Check the report for appropriate calculations.
5.13.4 For Sterility Test Laboratory investigation also check the following:
- Review the reports of a week prior to and after the day of test.
- Review the trends of last 2 months for any upward trend in microbial counts of the area.
- Check for the non viable particulate monitoring for any anomalies.
- Verify the results of negative product control test of the particular day.
- Review the sterility test report and verify whether all media and sterile materials are within expiration dates.
- Review the preparation record of all media, rinsing fluids and diluents used in the test.
- Verify the containers for sterility check if they are still under incubation or if any other similar lot are available for used.
- Review the differential pressure, temperature and relative humidity records for the day of test and near by days for any excursion observation.
- Review the instrument usage and maintenance logs and verify if any activities were performed that may have impact on the results.
- Impact of OOS on product quality shall be assessed by Head – QC & Head – QA or their Designee.
- Head – QC/Designee shall assess the data generated from the investigation to identify the probable cause.
- If an assignable cause is identified for OOS result, Head – QC shall record details in Format No. SOP/2022/XXX/Fyy-00 .
5.13.8 Following are the actions to be completed:
- Details of assignable cause.
- Corrective actions to be taken.
- Repeat test on the remaining sample (Sample A) if sample is available or on the Original Sample.
- If this sample portion is not available, or is spoiled, use previously collected sample for analysis. If this also is not available re-sampling may be performed with prior approval of QA.
- If the results found passing, invalidate the OOS results. Report the repeat test results and close the OOS with justification.
- Preventive actions to be taken for future including training of concern personnel.
- Disposition action for sample / batch under investigation (with justification) shall be provided.
- Evaluate and determine if a corrective action is require, if yes, describe the corrective action and preventive action.
- Ensure that the corrective action if require is implemented at the earliest and in timely manner.
- If the assignable cause is found to be due to analyst error, analyst shall be re trained and re qualified (if required) for the analysis.
- All the results of analysis performed by the analyst during the period shall be thoroughly evaluated for the results.
- If required, reanalysis shall be performed by other qualified analyst.
- If the results for stability samples do not conform, check for the expiry for product. Check for the storage condition, previously generated stability data, previous related OOS investigation and product related results.
- If the results found failing QA person shall perform extended investigation (Full Scale OOS investigation-Phase II).
5.14 Full scale OOS Investigation/Phase II Investigation:
- If assignable cause is not identified in laboratory investigation, proceed as below:
- The Phase II investigation shall be initiated within three working days, after conclusion of Phase I (Laboratory investigation) is completed and approved.
- Extended Investigation shall be performed by using investigation tool eg. Fish bone or Ishikawa diagram or using tools of quality risk assessment.
- QA person shall perform Extended Investigation / Failure Investigation.
- Investigation shall cover following points but no limited to:
- Perform the extended investigation at ware house for receipt, sampling, storage and dispensing of material.
- Perform investigation in manufacturing, packing and holding of drug substance and drug products.
- During investigation cover personnel, machine, material, environment and methods.
- Include the manufacturing process related contamination and cross contamination issues during investigation .
- If investigation does not reveal manufacturing error and data form repeat analysis shows the the original OOS result arise from non assignable cause, it can be concluded that OOS is not representative of the batch.
- Whenever required an appropriate CAPA can be taken to avoid the recurrence.
- Perform the Full scale OOS Investigation-Phase II investigation as per Format No. SOP/2022/XXX/Fyy-00 .
5.15 Microbial Enumeration Test and Test for Specified microorganisms- (Full Scale OOS Investigation) Phase II investigation:
- If assignable cause is not identified in laboratory investigation, proceed for Manufacturing process related investigation:
- Scope of following investigation is for In-process tests, stability samples and finished product analysis or material processed in manufacturing area.
- Check Environmental monitoring records (temperature, humidity, pressure differential, settle plate count, active air sampling count, particle count, personal monitoring), especially for the day of manufacturing / processing.
- If one or more of these analytical activity is not carried out on the day of manufacturing / processing, review previous / last records / history for that test for that area / location.
- Check Cleaning & Sanitization record for the area.
- Check equipment qualification, validation, calibration, cleaning and sanitization record.
- Check for Operators error (training / any unusual operation carried out).
- Check raw material analysis records.
- Impact of OOS on product quality shall be assessed by Head – QA and QC or Designee.
- If assignable cause is not identified repeat the analysis by re-sampling with larger sample pool (Twice the original sample if required)
- The repeat testing shall be performed only for the parameter which is showing an OOS/non complying result.
- Repeat testing to be performed in duplicate with same sample quantity by same/other microbiologist by following the same procedure of MET and TSM.
- If still sample fails, reject the batch. This applies even if one of the results of duplicate analysis is failing.
- Further decision on the disposition of batch / material shall be taken by Head QA, if required in consultation with Corporate Quality
5.16 Microbial Enumeration Test and Test for Specified microorganisms- (Full scale OOS Investigation) Phase II investigation for Raw/Packing Materials analysis:
- If assignable cause is not identified in laboratory investigation, proceed for extended investigation.
- Further investigation shall be carried by Head QA, Head QC or Designee in coordination with Vendor or if required in consultation with Corporate Quality.
- If assignable cause is not identified repeat the analysis by re-sampling the batch with larger sample pool (Twice the original sample if required).
- The repeat testing shall be performed only for the parameter which is showing an OOS/non complying result.
- The repeat analysis shall be joint analysis with vendor or analysis in presence of vendor.
- If feasible the 1st Microbiologist (Microbiologist who performed the analysis earlier) shall perform joint analysis with vendor. The procedure for joint analysis shall be similar to that of initial analysis.
- If still sample fails, reject the batch. This applies even if one of the result is failing in joint analysis.
- Further decision on the disposition of batch/material shall be taken by Head QA,.
5.17 Water Analysis (Full scale OOS Investigation)- Phase II investigation:
- If assignable cause is not identified in laboratory investigation, proceed for Full Scale OOS Investigation. Inform Head QA/QC/Manufacturing/Engineering etc.
- Head Manufacturing shall take decision to quarantine the batches manufactured during the period starting from sampling of sample under question till the closure of the investigation.
- Scope of this investigation includes Potable water/Treated water/DM water/Purified water/Water for Injection and Pure Steam.
- Check Environmental monitoring records (temperature, humidity, pressure differential, settle plate count, active air sampling count, and particle count) if the excursion observed is in the Manufacturing or Microbiology Lab.
- If one or more of these analytical activity is not carried out on the day of manufacturing/processing, review previous/ last records/ history for that test for that area/location.
- Compare the cultural and microscopic characters of organism(s) in question to most common contaminant ( In-house Isolate). If new organism isolated then identify as per SOP on Microbial Identification and maintenance of culture library.
- Check cleaning & Sanitization record for the sampling area.
- Check for error in sampling or any unusual observations during sampling.
- Check water system validation, calibration, cleaning, and sanitization record trend data.
- Interrogate the personnel’s present on the day of the activity and check for any error.
- Check for operator’s error (training / any unusual operation carried out).
- Review upstream water treatment systems(e.g. Carbon beds used to remove chlorine from Raw water).
- Examine the microbial count data for other samples or sites in the system -port contamination vs. System contamination.
- Review efficacy if sanitization procedure and schedule.
- Inspect system preventive maintenance records. Evaluate impact on product.
- Inspect system for dead lags, proper sloping, and proper sample port design and location.
- Review data for generation and distribution system for potential trends.
- Review data for the flow rate, pressure and vent filter integrity wherever used.
- Verify integrity of sample collection and testing procedures (if required).
- If assignable cause is not identified, inform Head – D. M. Water Plant / Manufacturing/ Engineering to proceed for corrective action.
- Appropriate and applicable corrective actions may include but not limited to;
- Rigorous cleaning and sanitization of sampling area
- Water system sanitization.
- Circulation loop sanitization.
- Regeneration of water system.
- Revision of the sanitizing agent, selection application methods and frequencies if required.
- Increased surveillance of personnel practices, possibly including written procedure for aseptic methods and techniques to be followed.
- Review of microbiological sampling methods and techniques.
- Impact of OOS on product quality shall be assessed by Head – QA and QC.
- Once the corrective action is taken, analyze the sample (sampling point with OOS results / observations) for 3 days consecutive monitoring.
- The repeat testing shall be performed only for the parameter which is showing an OOS/non complying result.
- The analysis may be performed by available Microbiologist. Efforts shall be made that Microbiologist involved in initial testing (OOS results) shall contribute to maximum extent.
- If all 3 days consecutive samples passes. The OOS is closed with summary.
- In case of 2nd and 3rd day samples are failing take corrective actions and repeat monitoring till satisfactory results / observations for three consecutive monitoring (days) with QA permission.
- In case the point(s) fails repeatedly despite of corrective action, point specific corrective and preventive measures shall be considered by Head – QA, Manufacturing, Engineering and other concerned.
- Additional testing of products/samples under impact due to the OOS shall be carried out if required.
5.18 Environmental Monitoring (Full scale OOS Investigation)-Phase II investigation :
- If assignable cause is not identified in laboratory investigation, proceed for Full scale OOS Investigation.
- Scope of this investigation includes settle plate monitoring, active air sampling, swab sampling, contact plating results and compressed air, nitrogen sample monitoring results.
- Perform the identification if the counts which exceed specification limit as per CSOP on Microbial Identification and maintenance of culture library.
- Check for error in sampling or any unusual observations during monitoring.
- Check Environmental monitoring records (temperature, humidity, pressure differential).
- Review previous / last records / history for that test for that area / location.
- Compare the cultural and microscopic characters of organism(s) in question to most common contaminant (In-house Isolate).
- Check cleaning & sanitization record for the area.
- Check the qualification status of the HVAC/equipment/personnel as per the observation.
- Review the monitoring records for the preceding and the succeeding day and review of the trends for the area.
- Interrogate the personnel’s present on the day of the activity and check for any error.
- Review the training record of the personnel
- If assignable cause is not identified, inform Head – Manufacturing/ Engineering to proceed for corrective action.
- Appropriate and applicable corrective actions may include but not limited to.
- Rigorous cleaning and sanitization of the area.
- Cleaning and sanitization of equipment / instruments.
- Increased surveillance of personnel practices, possibly including written procedure for aseptic methods and techniques to be reviewed.
- Review the microbiological sampling methods and techniques.
- Additional training on gowning practices, if excursion observed for gown or glove monitoring shall be imparted.
- Other (based on observations and results / observations) action if required shall be taken.
- Impact of OOS on product quality shall be assessed by Head – QA and QC.
- Once the corrective active is taken, monitor the sample (sampling point with OOS results / observations) for 3 consecutive monitoring (days).
- The repeat sampling/ testing shall be performed only for the parameter which is showing an OOS/non complying result.
- The analysis may be performed by available Microbiologist. Efforts shall be made that microbiologist involved in initial testing (OOS results) shall contribute to maximum extent.
- If all three consecutive samples passes. The OOS is closed with summary.
- In case sample fails any day during close monitoring (three consecutive days) repeat corrective actions and monitoring till satisfactory results / observations for three consecutive monitoring (days) with QA permission.
- In case the point(s) fails repeatedly despite of corrective action, point specific corrective and preventive measures shall be considered by Head – QA, QC, Manufacturing, Engineering and other concerned.
- Additional testing of products/samples under impact due to the OOS can be carried out if required.
5.19 Bacterial Endotoxin Testing (Full Scale OOS Investigation)-Phase II investigation :
- If assignable cause is not identified in laboratory investigation, proceed for Manufacturing process related investigation as per the below but not limited to :
- Check Cleaning & Sanitization record for the area.
- Check equipment qualification, validation, calibration, cleaning and sanitization record.
- Check for Operators error (training / any unusual operation carried out).
- Check raw material analysis records.
- Impact of OOS on product quality shall be assessed by Head – QA and QC.
- If assignable cause is not identified repeat the analysis by re-sampling.
- The repeat testing shall be performed only for the parameter which is showing an OOS/non complying result.
- Repeat testing to be performed in duplicate with same sample quantity by same/other microbiologist by following the same procedure.
- If Still sample fails, reject the batch. This applies even if one of the results of duplicate analysis is failing.
- Further decision on the disposition of batch / material shall be taken by Head QA.
5.20 Sterility Testing (Full Scale OOS Investigation)-Phase II investigation :
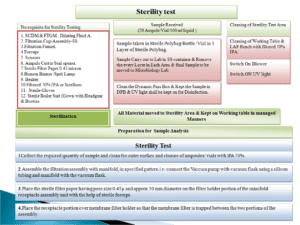

- If evidence of microbial growth is found, the product to be examined does not comply with the test for sterility, unless it can be clearly demonstrated that the test was invalid for causes unrelated to the product to be examined i.e. A clear failure due to laboratory reasons is identified.
- The test may be considered invalid only if one or more of the following conditions are fulfilled:
- The data of the microbiological monitoring of the sterility testing facility show a fault.
- A review of the testing procedure used during the test in question reveals a fault.
- Microbial growth is found in the negative controls.
- Identify the microorganism isolated from the test by using Strain typing method of identification (by genus and species level identification) or molecular fingerprinting (as feasible).
- Preserve tube/sample with results/observations in question.
- Subculture the growth on suitable media (e.g.: Bacterial or fungal growth promoting culture media).
- Record cultural and microscopic observations. Perform the identification up to species/ genus level of contaminant.
- If the growth is observed in FTGM perform sub culturing in duplicate incubate one set in aerobic conditions and one set in anaerobic conditions.
- If the contaminant is slow growing or does not grow on sub culturing to solid media use other methods to subculture and identify the organism.
- Identify the colonies from sterility testing area environmental and personnel monitoring plates sampled on the day of test if available or of subsequent days to determine the similarities.
- Determine the reason for the fault based on the source of microorganism either material or the technique followed in conducting the sterility test procedure
- Simultaneously proceed for Manufacturing/process Investigation
- Scope of following investigation is for in-process tests,stability samples and finished product analysis or material processed in manufacturing area.
- Check Environmental monitoring records (temperature, humidity, pressure differential, settle plate count, active air sampling count, particle count, drain monitoring), especially for the day of manufacturing/processing.
- If one or more of these analytical activity is not carried out on the day of manufacturing, review previous records for that test for that area/location.
- Compare the cultural and microscopic characters of organism(s) in question to most common contaminant ( In-house Isolate)
- Check cleaning & Sanitization record for the area
- Equipment validation, calibration, cleaning and sanitization and sterilization record. Review sterilization process.
- Check for operators error (training / any unusual operation carried out)
- Results/observations of in-process tests (if performed)
- If the test is declared to be invalid, repeat the test with the same number of units as in the original test.
- If no evidence of microbial growth is found in the repeat test, the product examined complies with the test for sterility.
- If microbial growth is found in the repeat test, the product examined does not comply with the test for sterility.This applies even if one of the result is failing in another microbiologist
- Further decision on the disposition of batch/material shall be taken by Head QA, if required in consultation with Corporate Quality.
- An extensive impact evaluation shall be done in case Sterility failure is observed and all the batches under a single campaign shall be tested.
- If the impact is for a large span the evaluation of batches from last successful process simulation study (media fill study) shall be taken under consideration.
5.21 General:
- Review, adequacy, applicability, implementation, accuracy and specificity of SOPs shall also be considered during investigation.
- The investigation is not limited to above mentioned procedures. If required investigations can be initiated for other tests as well if required.
- If for a test alert limit is not fixed or not defined then consider the action limit for the acceptance criteria.
- Whenever specification limits are not mentioned or the results are for information purposes only then the investigation is not required.
- Wherever and whatever is identified as probable/assignable cause, the part/activity shall be revised/modified/upgraded.
- Wherever possible and applicable results / observations of related samples shall be considered in full scale OOD investigation. This may include pre and post samples.
- Retesting and investigation may be focused to a particular test(s). e.g.: one of the test for specified microorganisms, only evaluation of Total aerobic microbial counts/Total viable aerobic counts or Total yeast and mold counts. Such decision shall be made by Head- QC with justification, in coordination with Head- QA.
- Similar procedure can be used with required customization for microbiological test not outlined in this procedures.
- Such procedure shall be checked by Head – QC and approved by Head – QA.
- In either of cases Training and re-training shall be considered by Head –QC & Head – QA , wherever necessary and applicable
- Laboratory investigation except microbial identification shall be completed within 7 days.
- Closer of complete OOS Investigation shall be performed within One month, In case extension of investigation is required justification for the same shall be duly approved from QA in Format No.
- NOTE: Excursion/OOS in bioburden evaluation of coating solutions, hold time study samples, cleaned and uncleaned equipment hold time study and samples other than routine samples shall not be investigated.
- Based on the results the time line/hold time period shall be evaluated and finalized.
- Excursion/OOS observed in samples sent to Contract testing laboratory shall be logged within 24 hrs after the receipt of the intimation from the laboratory.
- The laboratory shall be instructed to perform the relevant preliminary laboratory investigation and share the details within 7 days of the notification.
- Full scale OOS Investigation-Phase II investigation shall be initiated upon receipt of the laboratory investigation.
6.0 ANNEXURE (S):
Nil
7.0 ABBREVIATION(S):
-
OOS : Out of Specification
-
BET : Bacterial Endotoxin Test
-
QA : Quality Assurance
-
QC : Quality Control
-
MET & TSM : Microbial Enumeration Test & Test for Specified microorganisms
-
MDD : Microbiological Data Deviations
-
PLIR : Preliminary Laboratory Investigation report
-
HVAC : Heating Ventilation and Air conditioning
8.0 RECORD(S):
Name of Document Format No Originator Retained By Mode of filing
9.0 REFERENCE(S):
Investigating Out of specification (OOS ) test results for Pharmaceutical Production -USFDA Guidance Oct. 2006
Procedure for treatment of OOS results-EU Annex 4
Out of Specification guidance MHRA
Pingback:-


Very Incredible content
Very nice content
I am impressed with this web site, very I am a fan.
Hello there! I could have sworn I’ve been to this site before but after checking through some of the post I realized it’s new to me. Anyways, I’m definitely glad I found it and I’ll be bookmarking and checking back often!