Microbiologist Qualification:-

Analyst/Microbiologist Qualification in Sterility & Bacterial Endotoxin Test:
DEFINITIONS
Analyst Qualification: The analyst qualification is a program that establishes, cultivate and sustain the necessary skills to provide quality data collection.
- OBJECTIVE
- To describe the procedure of Analyst Qualification For Microbiological Analysis.
- SCOPE
- This SOP shall be applicable for analyst qualification for microbiological analysis at Microbiology Department .
- RESPONSIBILITY
- Microbiology Personnel shall be responsible for analyst qualification for microbiological analysis.
- Qualified officer microbiology or above who is trained and well versed with the procedure shall be responsible for analyst qualification for microbiology analysis.
- Head – Microbiology/ Designee shall be responsible for the compliance of the procedure.
- ACCOUNTABILITY
- Head – Microbiology/ Designee shall be accountable for the implementation of SOP.
- PROCEDURE
- Analyst should be trained for respective SOP of the test.
-
- On the first day of microbiologist qualification, firstly qualified microbiologist perform the analysis and then trainee microbiologist perform the analysis.
-
- Qualification activity should be performed under the supervision of trained/ qualified microbiologist or Head-Microbiology/ Designee.
-
For Sterility Testing:
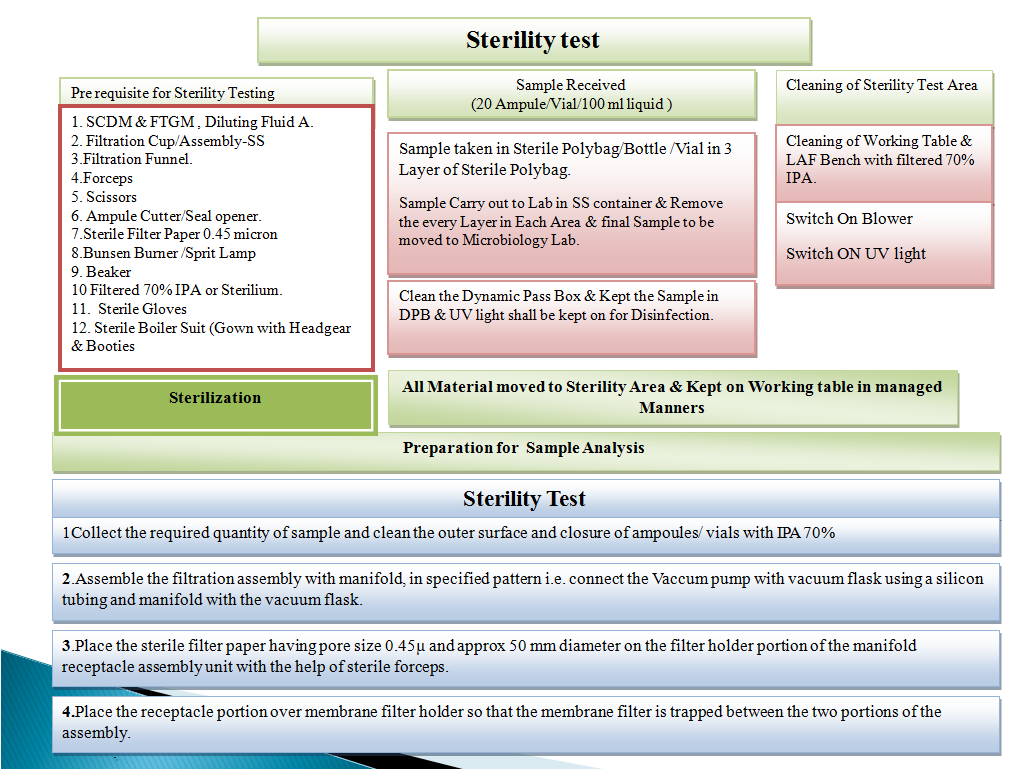

- Following items shall be required before execution of analyst qualification for Sterility testing:
-
-
- Sterile Water for Injection
- Laminar Air Flow
- Sterile Forceps & Scissor
- Sterile Soyabean casein Digest Medium
- Sterile Fluid Thioglycollate Medium
- Microbial Culture Suspension
- Filtration Assembly
- Sterile Membrane filter (0.45 µm and 47 mm)
-
-
- Preparation of Sample Vial:
-
-
- Prepare 100 X 100 mL Water for injection Tubes.
- Sterilize the tubes in autoclave using standard cycle.
- Segregate the sterilized tubes into group containing 20 tubes in each group.
- Give the identifications to each group as A, B, C, D and E.
- Contaminate one or two tubes in any one or two groups with NMT 100 CFU/mL of any one of the following cultures using a micropipette in Bio-safety Cabinet.
-
-
Note: Spiking (contamination) shall be done by In charge-Microbiology and shall keep spiking information sheet confidential till completion of the test. So, that the analyst shall not know the spiking information till completion.
-
-
- Record the activity as per formats.
- Perform the sterility test for all five groups of sterile water for injection using membrane filtration method following the procedure mentioned in the respective SOP. Culture related activity shall be performed in Bio-safety cabinet in Micro Lab-2. Perform sterility test of previously approved batch and negative control test as per respective SOP.
- After completion of incubation observe the tube. Growth should be observe in Positive control and spiked tubes and growth should be absent in negative control.
- Perform these tests on 3 consecutive days to confirm the reproducibility of the analyst performance.
- Record the activity as per Formats.
-
- Acceptance criteria:
-
- The un-spiked groups (3 or 4 groups) of sterile water for injection samples should pass the sterility test in all the three trials.
- The spiked group (1 or 2 group) of sterile water for injection samples should fail the sterility test in all the three trials.
- After successful completion of the test, a certificate shall be prepared for the approval of analyst qualification as per formats.
-
-
For Bacterial Endotoxin Test:
- Following items shall be required before execution of analyst qualification for Bacterial Endotoxin testing:
- LAL reagent
- CSE
- LRW
- Vortex mixer
- Depyrogenated 10 X 75 mm glass test tubes
- 12 X 75 mm depyrogenated borosilicate glass tube
- Calibrated heating block
- Calibrated micropipette
- Endotoxin free tips
- Test tube stand
- Following items shall be required before execution of analyst qualification for Bacterial Endotoxin testing:
- Preparation of Control Standard Endotoxin:
-
- Take out the CSE vial from the refrigerator.
- Wait for a few minutes so as to bring the vial in room temperature.
- Note down the details provided on the vial such as Mfg. date, Exp. date and lot number.
- Note down the potency of the vial.
- Reconstitute the vial as per supplier COA e.g. having a potency of 10000 EU/ vial with 4.0 mL of LRW.
- Perform the serial dilutions as given below to obtain a concentration of 8λ, 4λ, 2λ, λ, λ/2 and λ/4.
Tube No. Volume of Endotoxin Indicator solution Volume of LRW added Concentration
after dilution
Total
Dilution
01. 0.1 mL from re-constituted vial or ampoule 0.9 mL 250 EU/mL 1:40 02. 0.1 mL from Tube No.1 0.9 mL 25 EU/ mL 1:400 03. 0.1 mL from Tube No.2 0.9 mL 2.5 EU/ mL 1:4000 04. 0.2 mL from Tube No.3 1.8 mL 0.25 EU/ mL (2λ) 1:40000 05. 1.0 mL from Tube No.4 1.0 mL 0.125 EU/ mL (λ) 1:80000 06. 1.0 mL from Tube No.5 1.0 mL 0.06 EU/ mL (0.5λ) 1:160000 07. 1.0 mL from Tube No.6 1.0 mL 0.03 EU/ mL (0.25λ) 1:320000
-
-
-
- Perform each dilution with depyrogenated tips.
- Vortex for 1.0 minute after each dilution.
-
- Preparation of Lysate:
-
- Take out a fresh vial of Lysate from the refrigerator and allow it to acclimatize with the room temperature.
- Before opening the vial, collect LAL powder into the bottom of the vial by tapping on a hard surface.
- Place the rubber stopper on the work table in an inverted position, so that the inner surface of the stopper does not touch the surface.
- Re-constitute the vial according to the label claim with the LRW just before use by pipetting it directly in to the vial.
- While addition of LRW take care so as to avoid formation of air bubbles in the vials.
- Cover the vial with the original rubber stopper.
-
- Determination of Lysate sensitivity:
-
- Take out the Depyrogenated test tubes from Hot air oven.
- Perform the test on four standard CSE dilutions, i.e. 2λ, λ, λ/2 & λ/4 in quadruplicate and include negative control.
- Label the test tubes as 2λ, λ, λ/2 and λ/4.
- Mark all the reaction tubes as required and pipette out 100 μL of all the 4 CSE dilutions (2λ, λ, λ/2 and λ/4) in quadruplicate.
- In two test tubes, pipette out 100 μL of LRW (NWC).
- Add 100 μL of reconstituted LAL in all the test tubes.
- Incubate all the tubes at 37 ± 1 °C for 60 ± 2 minutes.
- After completion of incubation time, take out reaction tubes from heating block and invert it to 180° in one single smooth motion.
- If a firm gel has formed that remains in place upon inversion, record the result as positive.
- If an intact gel is not formed and falls down upon inverting the tube, record the result as negative.
- The test is invalid in following case:
- The lowest concentration of the standard solutions (λ/4) shows a negative result and the highest concentration (2λ) shows a positive result in all replicate tests.
- If negative control is positive.
-
- Determine the Geometric mean of end point concentration as follows:
Geometric mean End point concentration = Antilog (Σe)f
Where (Σe) = Sum of log of end point of all the replicates
f = Number of replicates
-
-
- Geometric end point concentration is the measured sensitivity of the LAL reagent in EU/mL.
- Perform these tests on 3 consecutive days to confirm the reproducibility of the analyst performance.
-
- Acceptance criteria:
-
- λ/4 should be Negative in all replicates.
- The end point result should be between 2λ and λ/2.
- Record all the result in formats.
- After successful completion of the test, a certificate shall be prepared for the approval of analyst qualification as per Formats.
-
6.ABREVIATIONS
-
-
- SOP : Standard Operating Procedure
- mm : Millimetre
- µ : Micron
- mL : Millilitre
- CFU : Colony Forming Unit
- NMT : Not More than
- ATCC : American Type Culture Collection
- LAL : Limulus Amoebocyte Lysate
- CSE : Control Standard Endotoxin
- LRW : LAL Reagent Water
- MFG: Manufacturing
- EXP: Expiry
- COA : Certificate of Analysis
- EU : Endotoxin Unit
- μL : Micro litre
- °C : Degree Celsius
- % : Percentage
- IPA : Iso Propyl Alcohol
- v/v : Volume by Volume
- MB : Microbiology
- NWC : Negative Water Control
-
7. ANNEXURES:
NIL
8. DISTRIBUTION:
MB
9. REFERENCES:
NA
10. REVISION HISTORY:
New
Pingback:-



It’s so much helpful for microbiologist,to clear ours problems.
This is my first time pay a quick visit at here and i am really happy to read everthing at one place
I loved even more than you will get done right here. The picture is nice, and your writing is stylish, but you seem to be rushing through it, and I think you should give it again soon. I’ll probably do that again and again if you protect this hike.
I am not sure where youre getting your info but good topic I needs to spend some time learning much more or understanding more Thanks for magnificent info I was looking for this information for my mission
My brother recommended I might like this web site He was totally right This post actually made my day You cannt imagine just how much time I had spent for this information Thanks
Somebody essentially lend a hand to make significantly posts I might state That is the very first time I frequented your web page and up to now I surprised with the research you made to create this particular put up amazing Excellent job
helloI really like your writing so a lot share we keep up a correspondence extra approximately your post on AOL I need an expert in this house to unravel my problem May be that is you Taking a look ahead to see you
Appreciating the commitment you put into your blog and detailed information you provide. It’s great to come across a blog every once in a while that isn’t the same outdated rehashed material. Great read! I’ve bookmarked your site and I’m adding your RSS feeds to my Google account.