FDA 483 WARNING LETTER :
A Form FDA 483, officially titled “Notice of Inspectional Observations,” is a document used by the U.S. Food and Drug Administration (FDA) during inspections of regulated facilities. The purpose of this form is to communicate to the inspected entity, typically a pharmaceutical, biotechnology, or food manufacturing facility, any observed deviations from the current Good Manufacturing Practice (cGMP) regulations.
Here are key points about FDA Form 483:
- Issuance: The FDA issues a Form 483 at the conclusion of an inspection if the investigators find conditions or practices that may violate regulatory requirements.
- Observations: The form contains a list of observations, which are specific deviations, deficiencies, or violations noted during the inspection. Each observation is typically numbered and includes a description of the observed issue.
- Communication: The issuance of Form 483 is a way for the FDA to communicate its initial findings to the facility’s management. It is not a final agency determination but highlights areas that may need corrective action.
- Response Required: The recipient of the Form 483 is usually given an opportunity to respond to the observations in writing. The response should include a plan for corrective action to address the noted issues.
- Regulatory Implications: While the 483 itself does not constitute a final agency action, it serves as a precursor to potential regulatory actions. If the FDA is not satisfied with the corrective actions taken, it may escalate enforcement actions, such as issuing a Warning Letter.
- Public Access: The FDA may make the Form 483 and the facility’s response available to the public through the Freedom of Information Act (FOIA).
- Corrective Action: The facility is expected to promptly and thoroughly address the observations outlined in the Form 483. Failure to take appropriate corrective action may lead to further regulatory consequences.
It’s important to note that receiving a Form 483 does not necessarily mean that a facility is in violation of the law, but it indicates areas where improvements are needed to ensure compliance with regulatory standards. The facility’s response and corrective actions play a crucial role in the resolution of the inspection findings.
Intas FDA Warning LaterWL_231122_121246
Read more:……….
Fundamentals of Microbial Limit Test 2023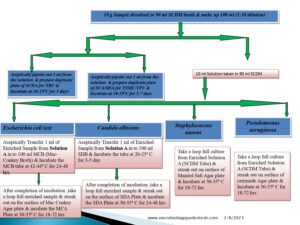


I was recommended this website by my cousin I am not sure whether this post is written by him as nobody else know such detailed about my trouble You are amazing Thanks
I have been browsing online more than three hours today yet I never found any interesting article like yours It is pretty worth enough for me In my view if all website owners and bloggers made good content as you did the internet will be a lot more useful than ever before
Its like you read my mind You appear to know a lot about this like you wrote the book in it or something I think that you could do with some pics to drive the message home a little bit but instead of that this is fantastic blog An excellent read I will certainly be back
I do believe all the ideas youve presented for your post They are really convincing and will certainly work Nonetheless the posts are too short for novices May just you please lengthen them a little from subsequent time Thanks for the post
I just could not depart your web site prior to suggesting that I really loved the usual info an individual supply in your visitors Is gonna be back regularly to check up on new posts
Wonderful web site Lots of useful info here Im sending it to a few friends ans additionally sharing in delicious And obviously thanks to your effort
Thank you for the auspicious writeup It in fact was a amusement account it Look advanced to far added agreeable from you However how can we communicate
For the reason that the admin of this site is working, no uncertainty very quickly it will be renowned, due to its quality contents.
I’ve been a loyal follower of this amazing website for the last few days. The brilliant content provided to users shows the site owner’s dedication. I’m extremely impressed and hope they continue posting such excellent content.
I think other website owners should take this site as an example , very clean and fantastic user pleasant design.