Disinfectant Efficacy test, antiseptics and other sanitizing agents.
Definition:,
Disinfectant: A chemical or physical agent that destroys or removes vegetative forms of harmful microorganisms when applied to surface.
Antiseptic: An antiseptic is a substance which inhibits the growth, or destroys microorganisms on living tissue including skin, oral cavities and open wounds and inhibits the development of microorganisms.
Sanitizing Agents: An agent for reducing, on inanimate surfaces, the number of all forms of microbial life including fungi, viruses and bacteria.
Sanitization :Destruction of most microorganisms (whether or not pathogenic) on wounds, clothing, or hard surfaces, through the use of chemicals or heat.
Chemical Disinfectant: A chemical agent on inanimate surfaces and objects to destroy infectious fungi, viruses and bacteria, but not necessarily their spores. Sporicidal and antiviral agents may be considered a special class of disinfectants. Disinfectants are often categorized as high level, intermediate level and low level by medically oriented groups based upon their efficacy against various microorganisms.
Cleaning Agent: An agent for the removal from facility and equipment surfaces of product residues that may inactivate sanitizing agents or harbor microorganisms.
Decontamination: The removal of microorganisms by disinfection or sterilization.
Sporicidal agent: An agent that destroys bacterial and fungal spores when used in sufficient concentration for a specified contact time.
Sterilants : An agent that destroys all forms of microbial life including fungi, viruses and all forms of bacteria and their spores. Sterilants are liquid or vapor phase agents.


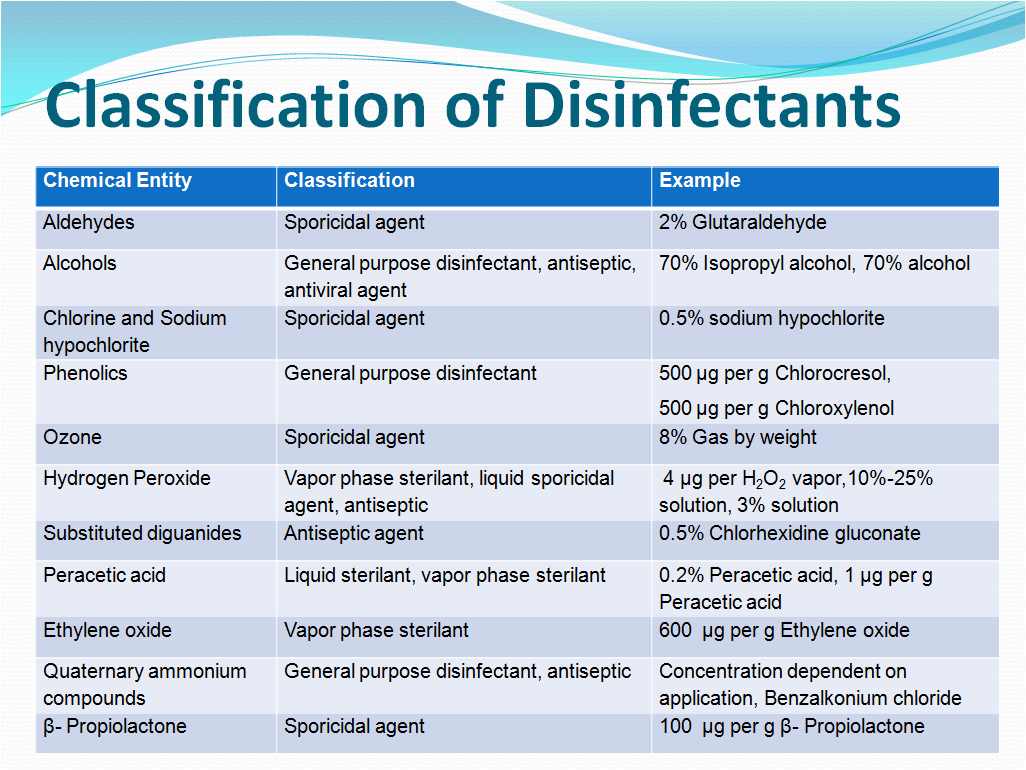
Classification of Disinfectants
Chemical disinfectants are classified by their chemical types. Following are the various types of disinfectants used with examples.
Selection, Mechanism, Concentration of a Disinfectant for Use :
Following points shall be considered while selecting the disinfectant for routine usage but not limited to
- Number and types of microorganisms to be controlled
- Spectrum of activity of the disinfectant.
- The reputation of the disinfectant supplier.
- Application method and contact time of the disinfectant.
- The nature of the surface material being disinfected.
- Compatibility with the disinfectant.
- The amount of organic compounds on the surface that may inactivate a disinfectant.
- Need to maintain residual bactericidal activity of the disinfectant on the surface.
- Corrosiveness of the disinfectant to equipment with the application.
- Safety considerations for operators applying the disinfectant.
- Compatibility of the disinfectant with the sanitizing agents and other disinfectants.
- The planned disinfectant rotation.
- Steps to be taken to avoid the contamination of pharmaceutical products by a disinfectant.
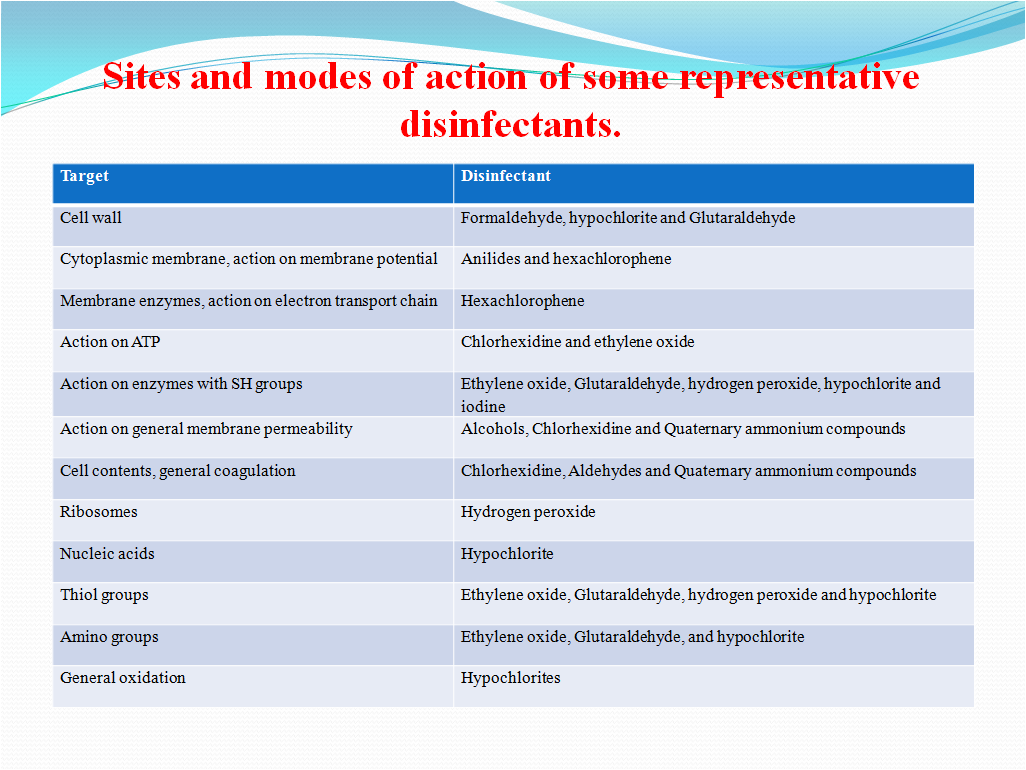
The sites and modes of action of some representative disinfectants.
Preparation of Disinfectant Test Dilution.
Prepare the dilution of disinfectant or sanitizing agent at which the effectiveness is to be checked, in sterile purified water.
Preparation of Inoculum of Test organisms:
Prepare the inoculum as per procedure on Microbial Culture maintenance and Enumeration.
Following cultures shall be taken for the various testing activities:
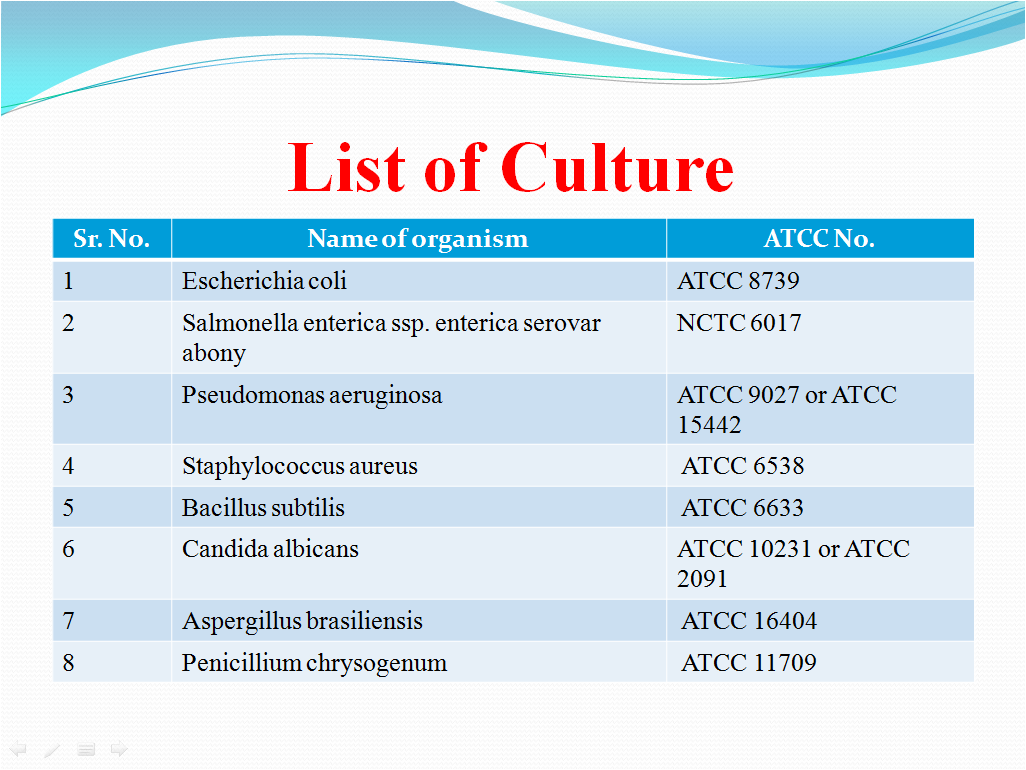
- In house Isolates (of different categories shall be used e.g. Gram positive, Gram negative organisms, Yeast or molds)
After incubation, dilute each microbial culture as per SOP on Microbial Culture maintenance and enumeration. - For obtaining spore suspension of Bacillus subtilis use SCDA media containing 0.35 g/L Manganese Sulfate (MnSo4.2H2O). Verify the culture prior to usage for the sporulation using spore staining method
- Phenol Coefficient Test (Rw Coefficient Determination):
Check the label of the disinfectant or get the details from the Manufacturer/supplier of the disinfectant for the Rw Coefficient/Phenol Coefficient value. - If the coefficient is not available then perform the test as mentioned below:
- Test Organism: Salmonella abony NCTC 6017
- Prepare a freshly inoculated (24 hr.) culture of the organisms for the test.
- Standard Phenol: 5% w/v solution in sterile purified water of chemically pure phenol shall be prepared.
- Test dilutions shall be prepared from the standard phenol by dissolving content equivalent to 1g of phenol in each 95, 100, 105, 115 mL of the solution made.
- The dilutions shall be used within a week of preparation.
- Test dilutions of Disinfectant (sample): Add 5 mL of the disinfectant into about 480 mL of sterile purified water and rinse the pipette two to three times or more using sterile purified water.
- Make up the volume to 500 mL with sterile distilled water.
- Mix the solution thoroughly.
Prepare appropriate dilutions as per the recommendation by the manufacturer of the disinfectant.
Procedure:
- Take 5 mL of 4 chosen dilution of the disinfectant in 4 test tubes, with the strongest dilution tube on the left.
- Place the tubes in water bath maintained at a constant temperature between 17-19ºC.
- Add to a test tube 5 mL of the particular phenol dilution and place it on the right.
- When all the tubes have reached the temperature of the water bath, consider the time as Zero time.
- Add 0.2 mL of the culture to the left hand test tube and shake the tube gently.
- After 30 seconds inoculate the next tube and repeat the process with each successive tube at intervals of 30 seconds until the phenol control tube is inoculated.
- Thirty seconds after this last addition (i.e. 2.5 minutes from the zero time)
- Withdraw a loop full of the well shaken content of the tube at the extreme left and inoculate in 5 mL of the broth medium or streak on the surface of DENA (Dey-Engley’s Neutralizing agar).
- Perform similar operation after thirty seconds for the second tube. Repeat this procedure at an interval of 30 seconds with each of the 5 test tubes working from the left to right until all 4 sets of culture have been made i.e. At 2.5, 5, 2.5 and 10 minutes after exposure.
- Ensure to take the loop vertically from the surface of the liquid with its plane horizontally and without touching the sides of the test tubes.
- Sterilize the loop by flaming between each operation and care shall be taken to cool before next usage.
- Incubate the inoculated broths or the plates for NLT 48 and NMT 72 hours at 35-37ºC.
- Observe the tubes/plates showing growth of the test organism. Record the details in Annexure.
- Calculate the coefficient by dividing the dilution of the disinfectant that shows growth of test organism in defined minutes but no growth thereafter by that dilution of the phenol which gives the same response.
E.g. The results may be as mentioned in the below table.
| Sample Disinfectant Dilutions | Time of Exposure in minutes | |||
| 2.5 mins | 5 mins | 7.5 mins | 10 mins | |
| 1:1000 | N | N | N | N |
| 1:1100 | P | N | N | N |
| 1:1200 | P | P | N | N |
| 1:1300 | P | P | P | N |
| Phenol Control (1:100) | P | P | N | N |
N: No Growth, P= Presence of growth
Rw Coefficient= 1200/100=12
Use of Neutralizers in Testing
- Neutralizers that inactivate the disinfectants shall be included in either the diluent or microbiological media used for microbial enumeration or both.
- Common neutralizing agent for common disinfectants are as below.
- If SCDA is used for enumeration purposes then use the following neutralizing agents in the diluents or add the agents in the medium prior to sterilization.
- Else use DENA medium or DEN Broth for the enumeration/dilution purposes.
| Disinfectant | Neutralizing agent |
| Alcohols | Dilution or polysorbate 80 |
| Glutaraldehyde | Glycine and sodium bisulfite |
| Sodium hypochlorite | Sodium thiosulfate |
| Chlorhexidine | Polysorbate 80 and lecithin |
| Mercuric Chloride and other mercurials | Thioglycolic acid |
| Quaternary ammonium compounds | Polysorbate 80 and lecithin |
| Phenolic compounds | Dilution or polysorbate 80 and lecithin |
Efficacy Testing : Tube method
- Inoculate individual separate tube of 100 mL of Disinfectant test dilution (prepared as per 5.4) with
 to
to  cfu/mL concentration of each microorganism
cfu/mL concentration of each microorganism - Monitor the viability of the each microorganism at contact period of 2 minute, 5 minutes and 10 minutes by aseptically taking 1 mL of disinfectant test dilution
- challenged with
 to
to  cfu/mL of microorganisms and inoculate in to 100 mL of liquid sterile culture media and incubate as mentioned below.
cfu/mL of microorganisms and inoculate in to 100 mL of liquid sterile culture media and incubate as mentioned below. - Bacterial cultures in Liquid culture medium (such as SCDM or DEN Broth) shall be incubated for a period of 14 days at 30-35ºC and Fungal cultures shall be incubated at 20-25ºC for a period of 14 days.
- After incubation observe visually the tubes for presence of growth by comparing turbidity with the negative controls (Sterile, Un-inoculated ) of same medium batch/lot.
- Record the result in format
- Indicate for presence of growth : P and Absence of growth: N
Positive Controls and Negative Controls
Positive Controls
- Inoculate 104 to 105 cfu of each microorganism in each separate tube of 100 mL of liquid culture media and incubate as mentioned in Pt. No. 5.8.3 with each test. This will serve as positive controls.
Negative Controls
- Keep sterile 100 mL of SCDM/DENB with each test as negative control.
Validity of the results:
Results are not valid in case:
- Positive Controls are not showing presence of growth.
- Negative controls are showing growth.
- If growth is absent in less contact time as compared to higher contact time showing presence of growth for same dilution of test disinfectant.
Interpretation of Results and Conclusion:
- The disinfectant test dilution will be considered effective if no growth is observed in 2 minute for any test microorganism.
- The disinfectant test dilution will be considered ineffective if growth is observed in any one of the time period for that contact time period for that specific test microorganism.
Use Dilution Test:
The test is carried out for screening disinfectants for their efficacy at various concentrations and contact times against a wide range of standard test organisms and environmental isolates.
Preparation of Culture Suspension:
Prepare the culture suspension of ![]() to
to![]() cfu/mL
cfu/mL
Preserve the culture as per validated time period for further usage.
Sample Preparation:
- Dilute the disinfectant with sterile purified water at ambient temperature from the stock so as to make the dilution used in areas. (Use Recommended Manufacturers Concentrations and half for lower dilution and double for upper dilution.)
- For e.g. If for a disinfectant the recommended concentration is 1% then the lower dilution would be 0.5% and the upper dilution would be 2%.
- Dispense 20 mL of the dilution into three sterile test tubes and label them as A, B and C. Prepare two such sets to get result in duplicate.
- Add 1 mL of culture containing
 to
to  cfu/mL of one of the microorganism into tubes A, B and C. Note down the time.
cfu/mL of one of the microorganism into tubes A, B and C. Note down the time.
Contact time establishment and recovery:
- Pre wet the filter using 100 mL of sterile 0.1% peptone water.
- After 2 minutes filter the solution from both the tubes labeled as “A” through a pre wetted 0.45 µ sterile cellulose nitrate membrane filter separately and give wash with 100 mL of sterile 0.1% peptone water to remove the traces of disinfectant to each of the membrane.
- Place the membrane on pre-incubated plate of sterile Soyabean Casein digest Agar with Lecithin and Tween 80 or use DENA (Dey Engley’s neutralizing agar)
- Repeat the above exercise to establish the contact time for 5 and 10 minutes for tubes B & C respectively.
- As positive control, add 1 mL of spike inoculum containing NMT 100 cfu/mL culture (after dilution from the stock culture of 105 to 106 cfu/mL) and filter the solution through 0.45 µ sterile cellulose nitrate membrane filter give a rinse of 100 mL with sterile 0.1% peptone water and place the filter on Soyabean Casein digest Agar with Lecithin and Tween 80 or (DENA medium).
- For Negative control, filter 20 mL sterile 0.1% peptone water through a pre wetted 0.45 µ sterile cellulose nitrate membrane filter and place the filter on Soyabean Casein digest Agar with Lecithin and Tween 80 or (DENA medium).
- Repeat the procedure for other micro-organisms as stated above.
- Label the plates
- Name of disinfectant,
- Name of the culture,
- Dilution,(or concentration of disinfectant)
- Contact Time,
- Date of Testing ,
- Microbiologist initial.
- Incubate all spiked with bacterial culture plates in inverted position at 30 to 35°C for 48-72 hrs and plates spiked with yeast and molds at 20 to 25°C for 3-5 days.
- After the incubation period, count the number of colonies on the membrane from all plates and note down the observation in Format
Acceptance Criteria:
- There shall be more than three (3) log reduction (vegetative cells) and two (2) log reduction (Spore formers) in the number of organisms selected for use dilution test.
Surface Challenge Test (Coupon Testing method):
- The test is carried out using standard test microorganisms and microorganisms that are typical environmental isolates, applying disinfectants to surfaces at the selected use concentration with a specified contact time and determining the log reduction of the challenge microorganisms.
Following surfaces can be selected based on the usage in the organization.
| Material | Application |
| Stainless Steel 304L and 316L grades | Work surfaces, filling equipment and tanks |
| Glass | Windows and vessels |
| Plastic, vinyl | Curtains |
| Plastic, poly carbonate | Insulation coating |
| Lexan (plexiglass) | Shields |
| Epoxy coated gypsum | Wall and ceilings |
| Fiberglass reinforced plastic | Wall paneling |
| Tyvek | Equipment wraps |
| Terrazzo tiles | Floors |
- Take 15 cm X 15 cm templates of each of the surfaces mentioned above for the study.
- Surfaces like SS,Glass shall be sterilized in drying oven /steam sterilization at 121ºC for NLT 30 mins.
- The surfaces which can not be sterilized by drying oven shall be disinfected using filtered 70% IPA, these surfaces are then taken to LAF dried and kept under UV in LAF for 15 minutes.
- Check for the initial Bio-burden on the surface as a control during the study.
- For SS & glass surface, double wrap the surface with aluminium foil.
- Sterilize the surface in drying Oven at 180°C – 190°C for two hours or at 250ºC for NLT 30 mins or by using steam sterilization at 121ºC for NLT 30 mins.
- Carry the surfaces to LAF bench and remove the aluminium foil carefully.
- Select /Mark 5 areas of 25 cm² on one surface template. Two areas shall be used for Bio-burden recovery, two shall be used for disinfectant validation and one shall be used as a negative control.
- Spread 0.1 mL of the cell suspension containing approximately
 to
to  cfu/mL of selected organism, on each template and allow drying for 20-30 minutes under the LAF.
cfu/mL of selected organism, on each template and allow drying for 20-30 minutes under the LAF. - Use two areas for initial count (bio-burden recovery from untreated surfaces) and the other two template for bio burden recovery from disinfectant treated surfaces.
You may also read article about Water for Injection (WFI)
Recovery from Control Surfaces:
- Aseptically remove a pre-moistened sterile swab taking care not to touch the swab head on anything except in the surface to be sampled. Follow CSOP on Environment monitoring for preparation, usage and testing of swabs.
Move the head of the swab slowly over the area to be tested. - Rotate the swab throughout the surface giving 06 time vertical and horizontal parallel strokes.
Place the swab to the sterile tube/container and replace the cap tightly. - Under LAF aseptically add 10 mL sterile 0.1% peptone water to the test tube/container and vortex.
- Dilute the above suspension appropriately and determine the Microbial load by the membrane filtration method for each dilution. Give the detailed dilutions for better understanding.
- For e.g. The culture inoculated is
 to
to  cfu/mL and 0.1 mL is inoculated on each surface then the inoculated concentration is
cfu/mL and 0.1 mL is inoculated on each surface then the inoculated concentration is  to
to  cfu/mL.
cfu/mL. - Consider the concentration as
 cfu/mL in swab tube when 10 mL sterile peptone water is added the concentration becomes as
cfu/mL in swab tube when 10 mL sterile peptone water is added the concentration becomes as  cfu/mL
cfu/mL - Prepare dilution 1: 10 Sterile Peptone water so that the next dilution is
 cfu/mL
cfu/mL - Next 1: 10 Sterile Peptone water so that the next dilution is
 cfu/mL
cfu/mL - Next 1: 10 Sterile Peptone water so that the next dilution is
 cfu/mL
cfu/mL - Arrange the filter assembly attach the vacuum pump and pre wet the filter and filter the content from individual tubes through separate 0.45μ x 47 mm membrane filter.
Give wash to each filter with 100 mL of pre-sterilized 0.1% peptone water. - Aseptically transfer the membrane filter on pre incubated media plates of sterile Soyabean Casein Digest Agar with Lecithin and Tween 80 or DENA medium.
Perform negative control by filtering 100 mL sterile 0.1% peptone water. - Incubate the plate(s) at 20-25ºC for NLT 3 days and then at 30 –35ºC for NLT 2 days in inverted position, appropriate precautions shall be taken so that integrity of the plate shall be maintained while transferring the plates from one incubator to another.
- After completion of incubation period observe the results and record as cfu / 25 cm2 in Format.
Recovery from Test Surfaces:
- Spray/wipe the surface (as per procedure followed at site) with the disinfectant at remaining two templates test surface(TS).
- Allow contact time between disinfectant and test organism (as per the time established during the use dilution study).
After this aseptically remove a pre-moistened sterile swab taking care not to touch the swab head on anything except in the surface to be sampled. - Rotate the swab throughout the procedure giving vertical and horizontal strokes.
- Place the swab to the sterile tube/container and replace the cap tightly.
- Under LAF aseptically add 10 mL sterile 0.1% peptone water to the test tube/container and vortex.
- Aseptically filter the content of the tubes through 0.45μ x 47 mm membrane filter.
- Rinse the tube/container containing swab with 2 portion each of 10 to 15 mL sterile saline solution and filter through same membrane give 3 washings of 100 mL each of pre-sterilized 0.1% peptone water.
- As a negative control, swab the un-inoculated area of 25cm2 from the selected surface and aseptically transfer the membrane filter on pre incubated media plates of sterile Soyabean Casein Digest Agar with Lecithin and/ Tween 80 or (DENA medium).
- Perform the procedure for swab testing by membrane filtration method and after rinsing the tube/container containing swab with 2 portion each of 10 to 15 mL sterile saline solution and filter through same membrane give 3 washings of 100 mL each of pre-sterilized 0.1% peptone water.
- Incubate the plate(s) at 20-25ºC for NLT 3 days and then at 30 –35ºC for NLT 2 days in inverted position, appropriate precautions shall be taken so that integrity of the plate shall be maintained while transferring the plates from one incubator to another.
- After completion of incubation period, observe the results and record as cfu / 25 cm2 in Format.
Acceptance Criteria:
- There shall be more than three (3) log reduction(vegetative cells) and two (2) log reduction (Spore formers) in the number of selected organisms after applying the disinfectant to surfaces such as Epoxy, SS plate,Glass,Teflon etc.



Great tremendous issues here. I?¦m very happy to peer your article. Thank you so much and i am taking a look ahead to contact you. Will you kindly drop me a mail?
An attention-grabbing dialogue is value comment. I think that you need to write extra on this subject, it might not be a taboo subject however generally persons are not sufficient to speak on such topics. To the next. Cheers