Sterility Test by Open Method
Sterility testing is a critical quality control procedure performed to determine the absence or presence of viable microorganisms in a pharmaceutical product, medical device, or other sterile preparations. The test is conducted to ensure that the product meets the required sterility standards and is free from microbial contamination that could pose a risk to patient safety.
Here are some key points regarding sterility testing:
- Purpose: The primary objective of sterility testing is to verify the effectiveness of the sterilization process and assess the product’s sterility. It is a crucial step in the quality control of sterile pharmaceuticals, including injectable drugs, ophthalmic preparations, parenteral nutrition solutions, and implantable medical devices.
- Test Methodology: Sterility testing typically involves inoculating the product or a sample from the product into a suitable growth medium that supports the growth of a broad spectrum of microorganisms. The sample is incubated under appropriate conditions for a specified period, which varies depending on the product and testing requirements. After incubation, the culture media are examined for the presence of microbial growth, indicating a positive result.
- Test Considerations: Sterility testing requires careful consideration of factors such as sample size, test method selection, validation of test methods, aseptic technique, and appropriate controls. Test methods may include membrane filtration, direct inoculation, or the use of automated systems. The choice of method depends on the product characteristics, regulatory requirements, and specific testing objectives.
- Regulatory Requirements: Sterility testing is often a regulatory requirement for pharmaceutical products and medical devices. Regulatory agencies, such as the United States Pharmacopeia (USP), European Pharmacopoeia (EP), or other national regulatory bodies, provide guidelines and requirements for sterility testing procedures. Compliance with these standards is essential for product registration, approval, and ensuring patient safety.
- Environmental Monitoring: Sterility testing is closely linked to environmental monitoring. The test evaluates the product’s sterility, but it is crucial to ensure that the testing environment itself is free from microbial contamination. Regular monitoring of the testing facility, including air and surface sampling, is performed to maintain a controlled environment and minimize the risk of false-positive results.
- Validation and Quality Control: Sterility testing methods and processes require validation to demonstrate their suitability and reliability. This includes validating the sterility test method, confirming the growth promotion ability of the culture media, and establishing appropriate controls. Additionally, robust quality control measures are implemented, including sterility testing of media, personnel training, and adherence to Good Manufacturing Practices (GMP).
It’s important to note that sterility testing has its limitations, and it is not possible to guarantee absolute sterility. The test can only detect viable microorganisms that can grow under the specific testing conditions. It is essential to combine sterility testing with other quality control measures, such as process validation, environmental monitoring, and good manufacturing practices, to ensure the overall sterility assurance of products.
Always consult relevant regulatory guidelines and follow established procedures and protocols when performing sterility testing to ensure compliance and accurate results.
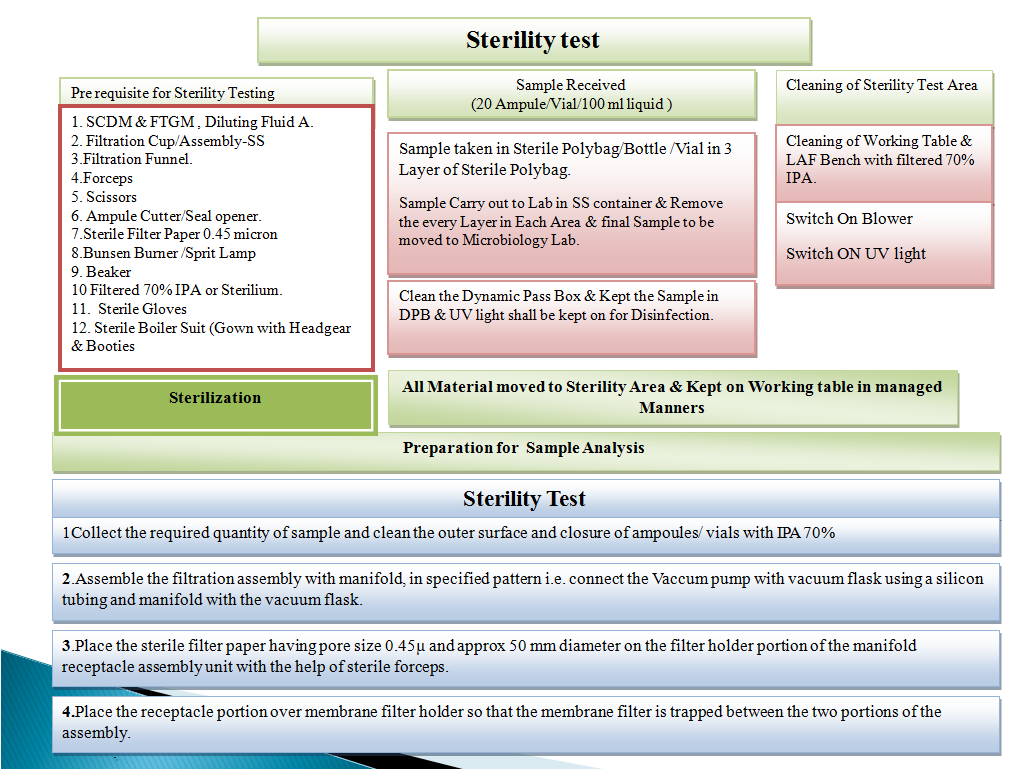
Flow diagram of Sterility Test:-


I precisely needed to appreciate you again. I am not sure what I would’ve undertaken in the absence of these techniques discussed by you on that concern. This was a frustrating situation for me, but being able to view the skilled tactic you dealt with the issue took me to jump over fulfillment. Extremely happier for the guidance as well as trust you really know what a powerful job that you are undertaking educating most people through the use of a blog. I am sure you’ve never got to know any of us.
I’m not sure exactly why but this blog is loading very slow for me. Is anyone else having this problem or is it a problem on my end? I’ll check back later on and see if the problem still exists.
I’ll immediately take hold of your rss feed as I can not to find your email subscription link or newsletter service. Do you’ve any? Kindly allow me recognise so that I may just subscribe. Thanks.