Growth Promotion Test.
The Growth Promotion test is a procedure used to confirm the ability of a culture medium to support the growth of microorganisms. This test is performed to ensure that the media used in microbiological testing is of good quality, and can reliably support the growth of microorganisms.
The Growth Promotion test involves inoculating the culture medium with a known strain of microorganisms, such as Escherichia coli or Staphylococcus aureus. The inoculated medium is then incubated under appropriate conditions, and the growth of microorganisms is monitored over a specified period of time.
If the microorganisms grow well on the medium, it is considered to be a good quality medium, and can be used for microbiological testing. If there is no growth or poor growth of microorganisms, it indicates that the medium may be contaminated or of poor quality, and should not be used for testing.
The Growth Promotion test is an essential quality control procedure in microbiology laboratories, as it ensures that the results obtained from microbiological testing are accurate and reliable.
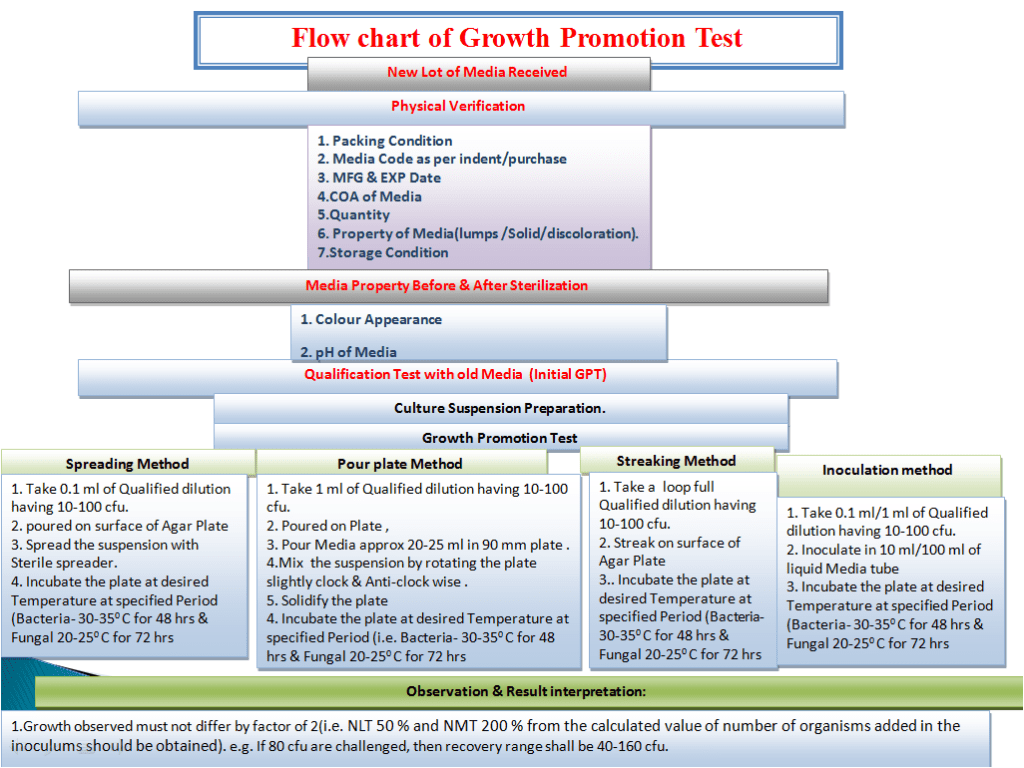
1.0 OBJECTIVE:
To lay down a procedure for Growth Promotion, Inhibitory and Indicative Properties of Culture Media Test.
2.0 SCOPE:
This SOP is applicable for Growth promotion test, Inhibitory and Indicative Properties of Culture Media Test at Microbiology Department
3.0 RESPONSIBILITY:
Officer / Executive – Microbiologist
4.0 ACCOUNTABILITY:
Head – MB
5.0 PROCEDURE:
5.1 GENERAL INSTRUCTION:
- Growth promotion test shall be carried out for all the newly received lot and prepared media. For new lot in addition to Growth promotion, Inhibitory and indicative properties shall also be determined.
- After receiving the new lot media, numbering shall be carried out for all the media containers as per SOP Media management.
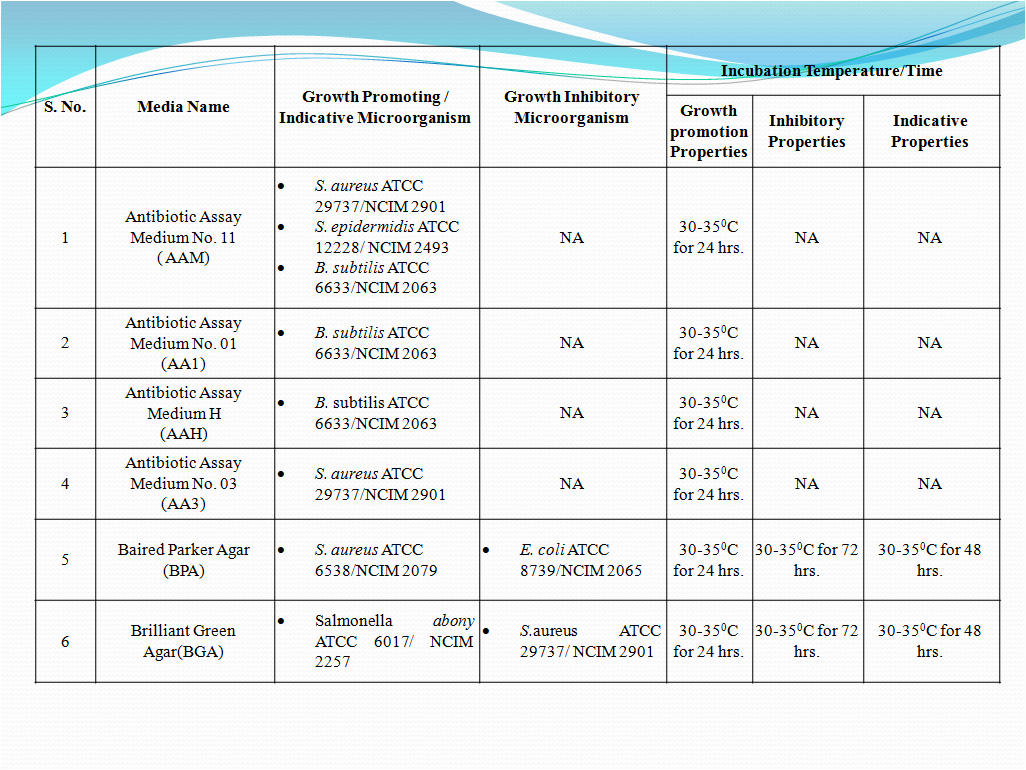
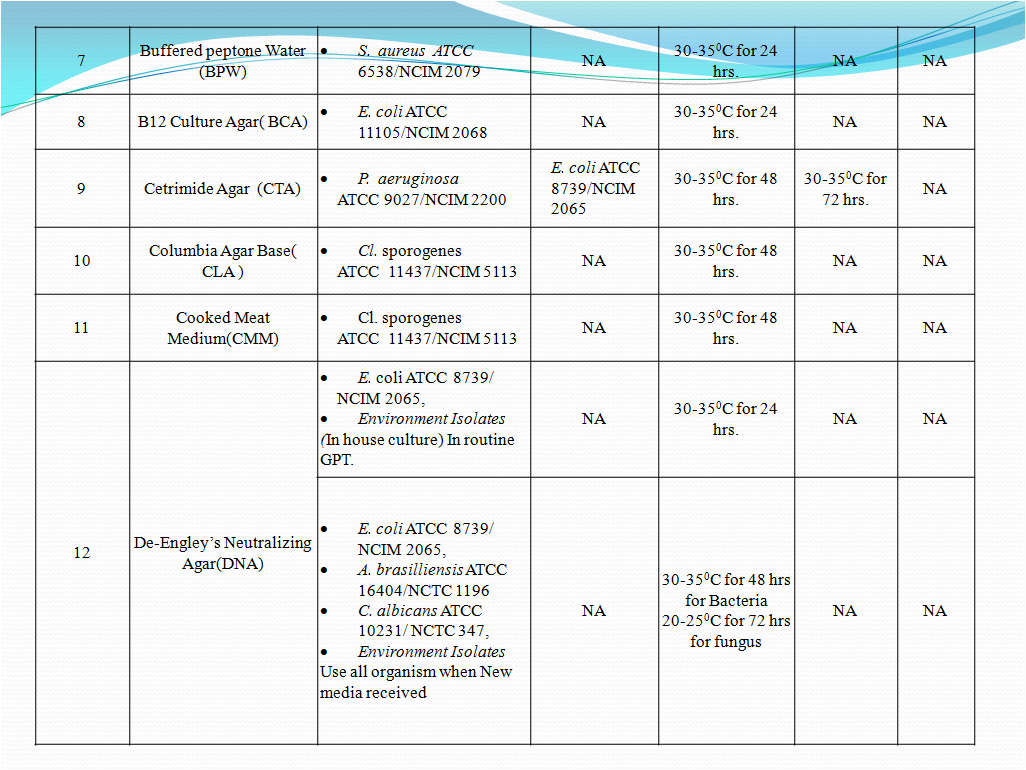
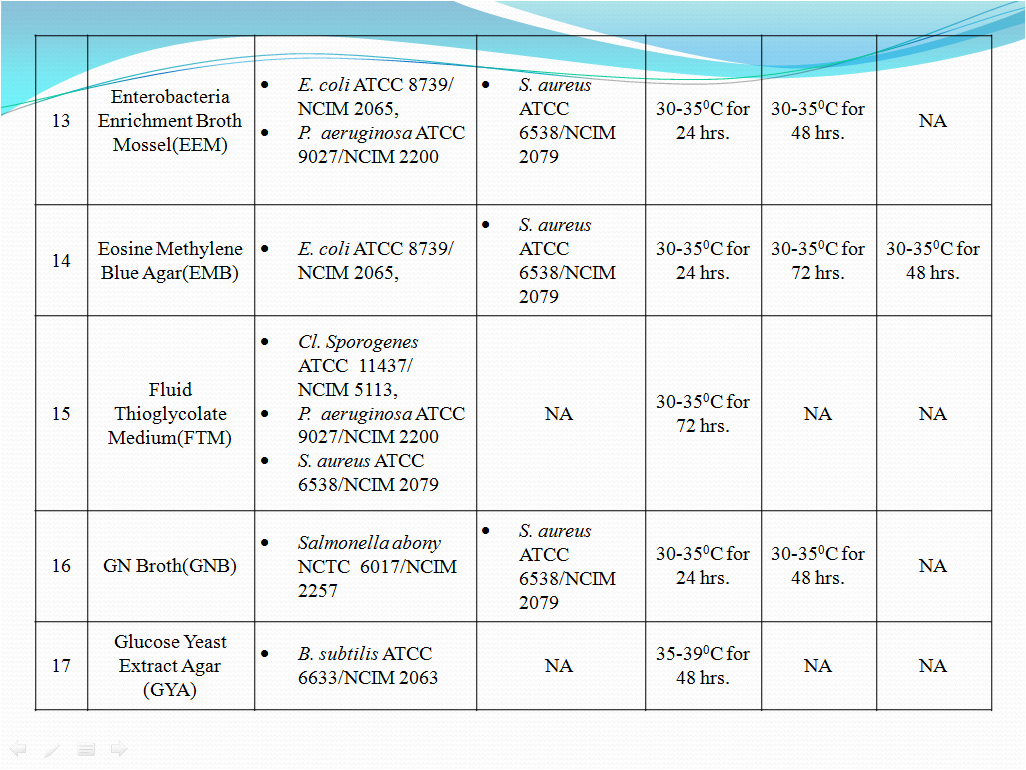
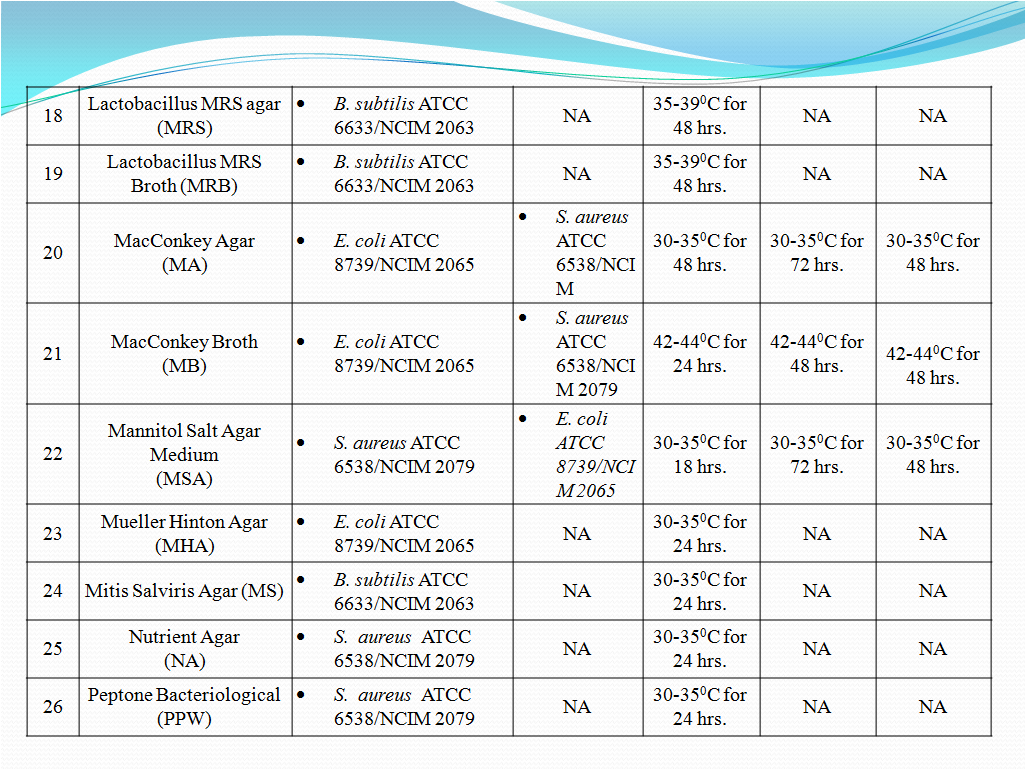
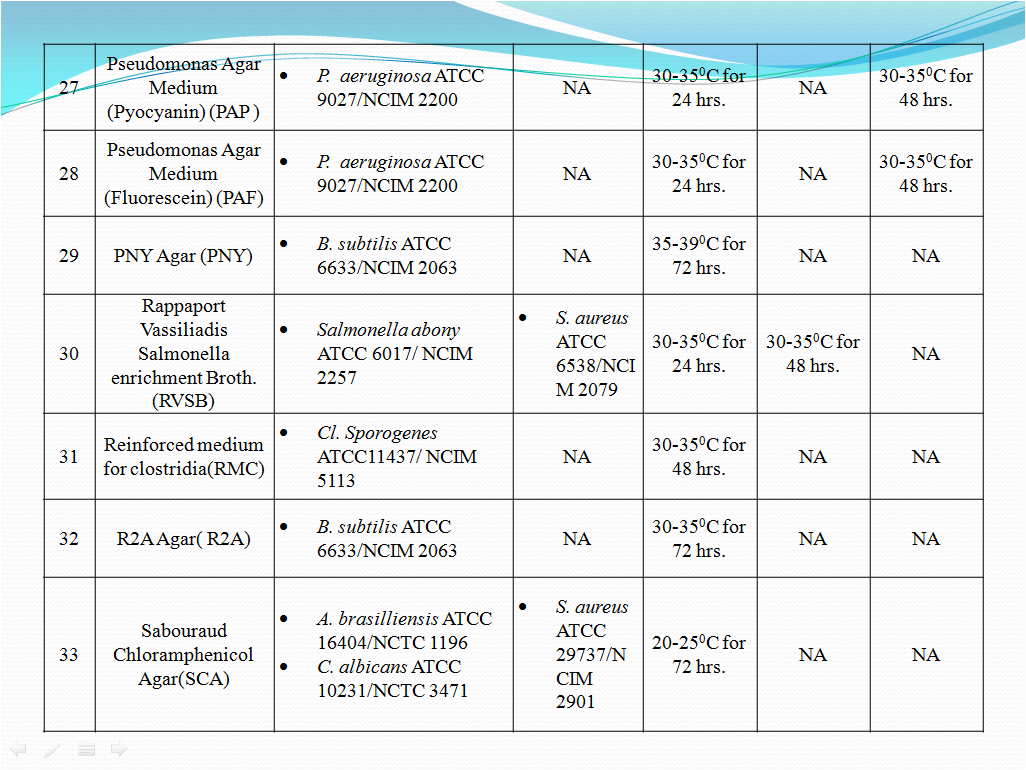
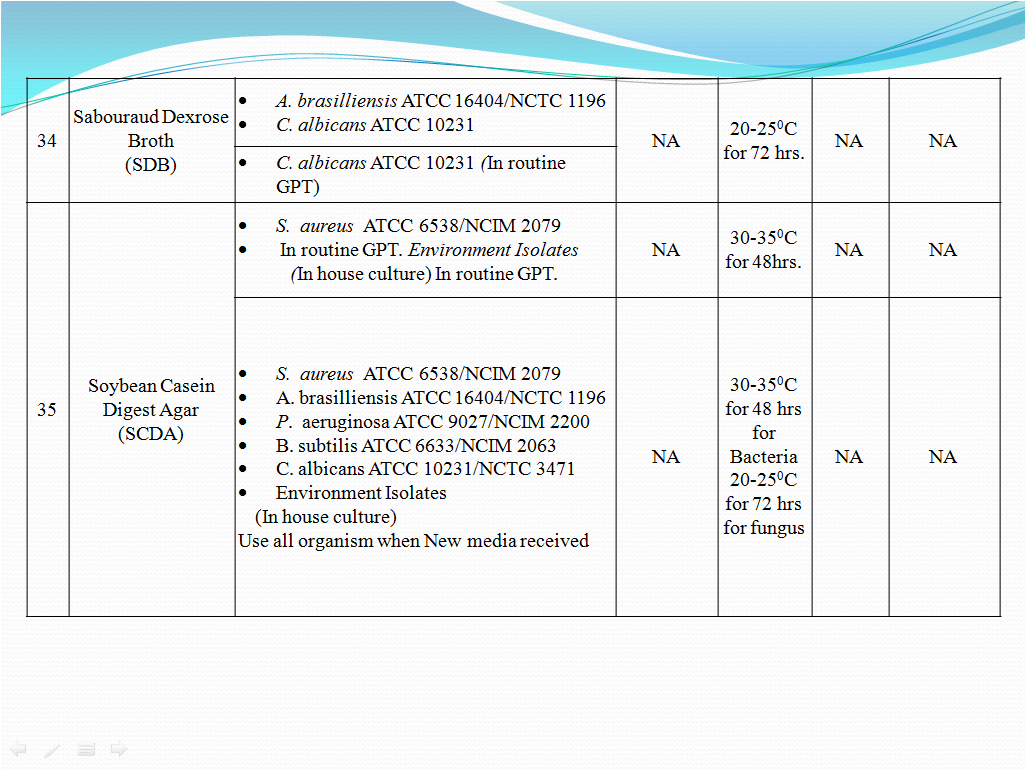
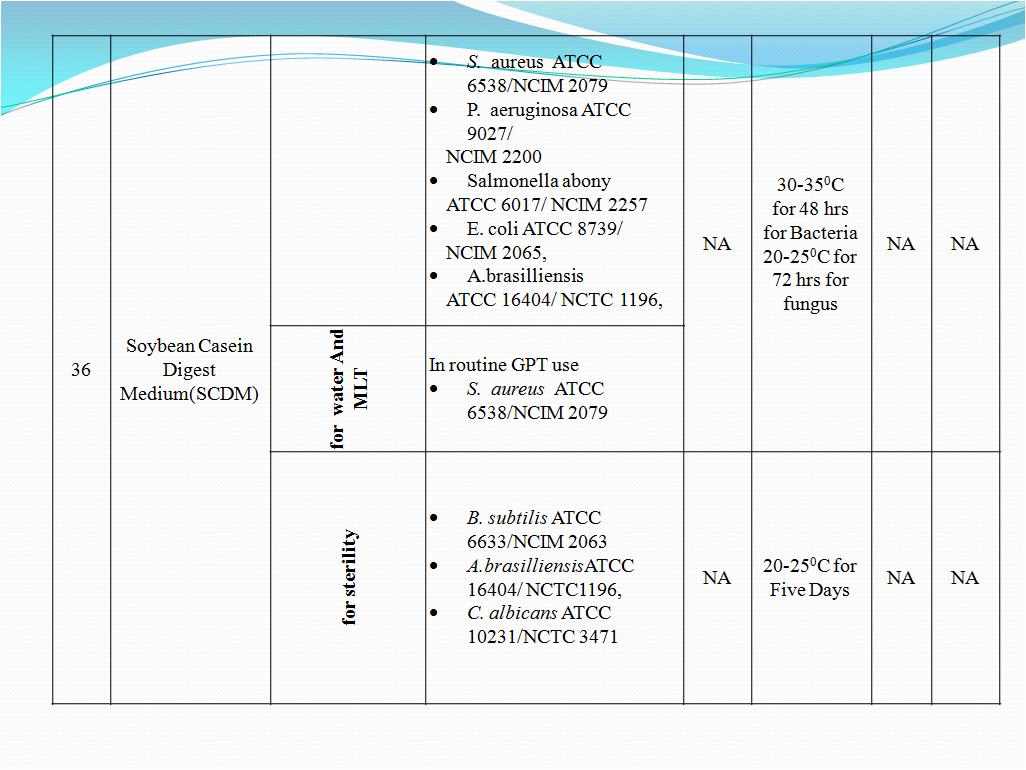
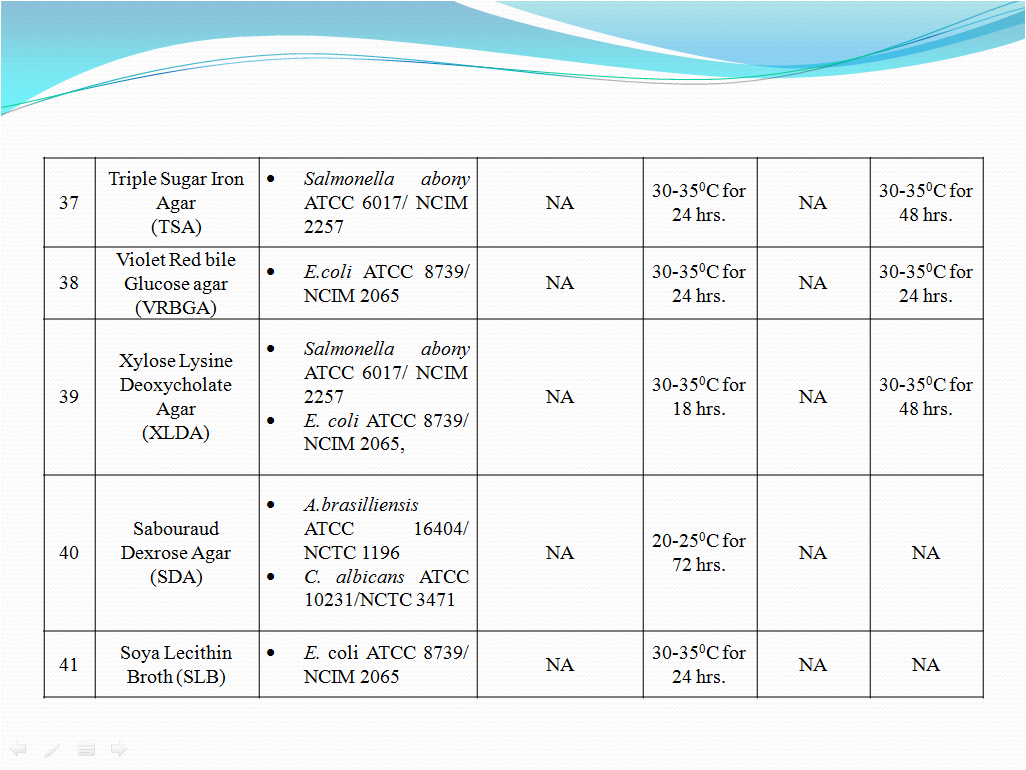
- To perform Growth promotion test on new media lot, take any one media container from the new lot and carry out test with microorganisms as shown in Table-I. If more than five organisms mentioned in Table-I then perform the Growth Promotion test with minimum five organisms including minimum one fungus.
- For new lot agar media, Growth promotion, Inhibitory and indicative test shall be carried out both quantitatively and qualitatively to determine the efficacy of media.
- For new lot broth media, Growth promotion, inhibitory and indicative test shall be carried out only qualitatively to determine the efficacy of media.
- Prepare the media for any of the above activity as per SOP Media Preparation
5.2 Growth Promotion Test For New Lot Agar Media ( Quantitative):
- For all the agar media, quantitative efficacy (i.e. percentage of recovery of organism) of media shall be determined either by pour plate or spread plate method.
- In pour plate method, inoculate 1 ml of any one specific culture suspension (having less than 100 CFU) in to sterile Petri plate as mentioned in Annexure 1.0 and add 15 -20 ml of media which has been maintained at not more than 55°C.
- In spread plate method, inoculate 0.1 ml of any one specific culture suspension (having less than 100 CFU) on particular media agar surface as mentioned in Annexure 1.0 and aseptically spread the inoculum with sterile spreader evenly.
- Incubate the media at particular temperature and time as mentioned in Table-I.








- Along with new lot media GPT, already approved media lot shall be tested for comparison study.
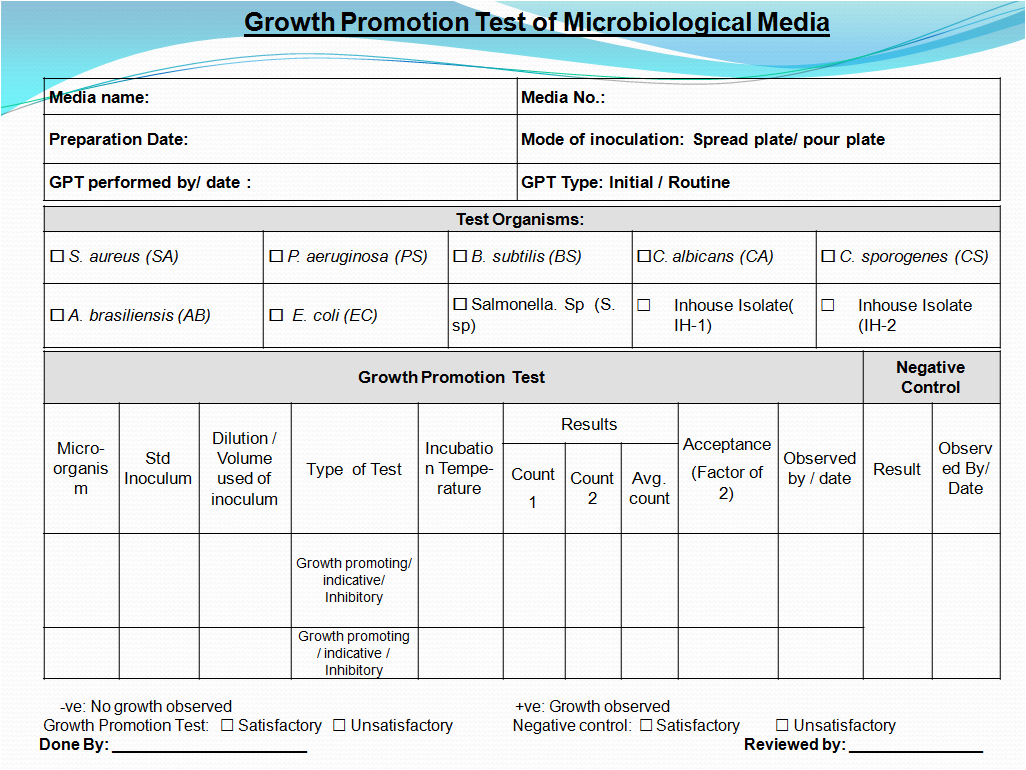
- After completion of incubation, record the details of observation in Report and calculate the percentage of recovery for new lot with compare to previously approved media. If any new media receiving for the first time, then inoculated microorganism shall be taken for calculation.

- In the test for Growth Promoting Properties microbial recovery for agar media growth obtained must not differ by a factor greater then 2 from the calculated value for a approved media lot or inoculated organism.
5.3 Inhibitory and Indicative Test For New Lot Agar Media (Qualitative):
- For selective agar media, qualitative efficacy (i.e. to determine its inhibitory property and indicative property of media on particular organism) shall be determined by streaking method.
- In streaking method, aseptically take a loopful of organism from culture suspension (having less than 100 CFU) as mentioned in Table-I for particular media and streak it on solidified agar surface of sterilized or prepared new lot media.
- Incubate the media at particular temperature and time as mentioned in Table-I.
- After completion of incubation, record the details of observation in Report.
- In new lot media similar characteristic growth as mentioned in Table-I should be observed in indicative property test.
- No growth should be observed as mentioned in Table-I in new lot media for inhibitory property test.
- For general purpose agar media only, Growth Promotion Test shall be carried out by inoculation method with exemption of inhibitory and indicative test.
- Along with new lot media, any approved media lot shall be tested with all test parameters for comparison study.
5.4 Growth Promotion, Inhibitory and Indicative Test for New lot broth Media:
- For selective broth media, qualitative efficacy (i.e. to determine its inhibitory property and indicative property of media for particular organism) shall be determined by inoculation method.
- Aseptically take a loopful of organism from culture suspension (having less than 100 CFU) as mentioned in Annexure 1.0 for particular media and inoculate it on sterilized or prepared new lot media.
- Incubate the media at particular temperature and time as mentioned in Table-I.
- After completion of incubation record the details of observation in Report.
- In new lot media, similar characteristic growth (i.e. change in media colour or turbidity) as mentioned in Table-I should be observed in indicative property test.
- No growth (i.e. no turbidity or colour change) should be observed in new lot media as mentioned in Table-I for inhibitory property test.
- For general purpose media only Growth Promotion Test shall be carried out by inoculation method mentioned above with exemption of inhibitory and indicative test.
- Along with new lot media, any approved media lot shall be tested with all test parameters for comparison study.
- Growth Promotion Test shall not be performed, if the same lot no. media received again.
5.5 Growth Promotion Test for Prepared Media:
- Growth promotion Test shall be carried out for all the prepared media with any one of the specific organism mentioned in Table -1.
- For broth media inoculate 1ml having less than 100 CFU of microorganism from culture suspension and incubate it at appropriate temperature and time period mentioned in Table -1.
- For agar media, take a loopful of organism and streak it on surface of media or carry out spread plate with 0.1ml of inoculum having less than 100CFU of microorganism from culture suspension and incubate it at appropriate temperature and time period mentioned in Table-1.
- Record all the details of Growth promotion and their results for prepared media in Report If characteristic Growth on prepared media observed as per Table-1, then write ‘Complies’ in GPT result column provided in the Report
- If the results of GPT indicate failure, discard the whole lot of prepared media and consider all the tests performed using that lot as invalid
- In GPT failure cases, again prepare fresh lot of dehydrated media from the same media container and perform GPT again.
5.6 ACCEPTANCE CRITERIA:
- In the test for Growth Promoting Properties microbial recovery for agar media growth obtained must not differ by a factor greater then 2 from the calculated value for a standardized Inoculum.
- For broth culture media luxurious growth of microorganism should be observed comparable to the previously obtained with previously tested and approved batch of medium occurs.
- In the test for Inhibitory Properties there should not be any growth of the microorganisms.
- In the test for Indicative Properties colony morphology and indication reaction should be similar to that obtained with the previously approved batch of media.
- If previous approved media is not available then media can be used in routine testing on the behalf of Vendor COA.
6.0 ABBREVIATIONS:
- No. Number
- QA Quality Assurance
- QC Quality Control
- SOP Standard Operating Procedure
- GPT Growth Promotion Test
7.0 ANNEXURES:
8.0 DISTRIBUTION:
MB
9.0 REFERENCES:
United State Pharmacopoeia 37
Indian Pharmacopoeia 2022, 2.2.9 (Biological Method).
10.0 REVISION HISTORY:
New

I like the helpful information you supply to your articles. I will bookmark your weblog and test once more right here frequently. I’m fairly certain I will learn plenty of new stuff right here! Good luck for the following!
My husband and i ended up being absolutely contented when Emmanuel could do his basic research because of the precious recommendations he came across through the blog. It’s not at all simplistic to just be offering techniques which usually other folks have been making money from. We really remember we’ve got the website owner to be grateful to for that. Most of the explanations you made, the straightforward site navigation, the relationships your site help instill – it’s mostly spectacular, and it’s really aiding our son and the family reckon that the issue is thrilling, which is certainly really serious. Thanks for the whole thing!
I simply could not depart your site before suggesting that I really enjoyed the standard information a person supply for your visitors? Is going to be back frequently in order to inspect new posts.
Thank you for the auspicious writeup. It in fact was a amusement account it. Look advanced to more added agreeable from you! By the way, how can we communicate?
I like what you guys are up too. This kind of clever work and exposure! Keep up the fantastic works guys I’ve incorporated you guys to blogroll.